Endocrine Regulation of Lipid Metabolism: How Hormones Shape the Lipid Profile
Lipid metabolism can be understood as comprising two interdependent components. The first is lipoprotein metabolism, which governs the transport and distribution of lipids between the liver, intestine, and peripheral tissues. The second is intracellular lipid metabolism, encompassing the synthesis, storage, and breakdown of lipids to provide energy locally within cells or to supply distant tissues. These two facets are tightly interwoven: intracellular lipid pathways generate substrates that enter the circulation via lipoproteins, while circulating lipids influence cellular lipid handling. If you haven’t done so already, I recommend reading the clinical pearl about the core concepts of lipid metabolism first.
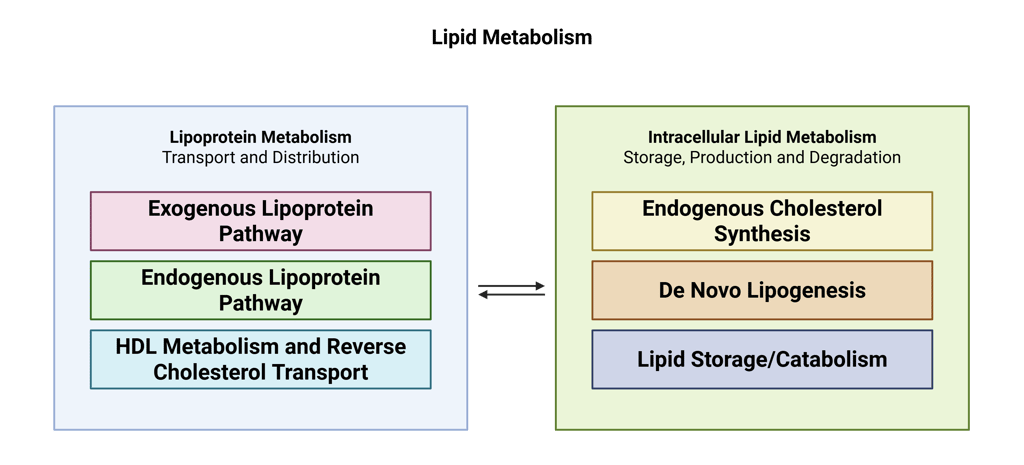
Overview of the two components of lipid metabolism
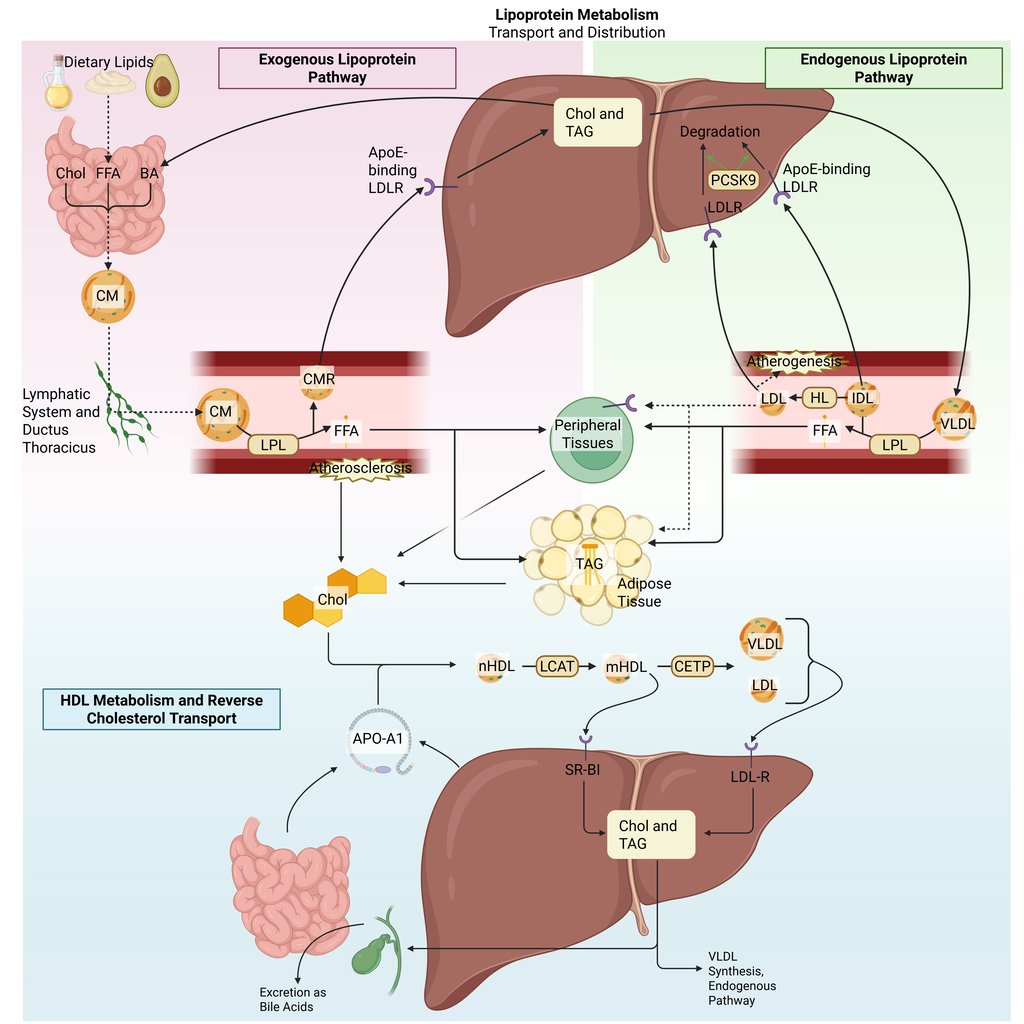
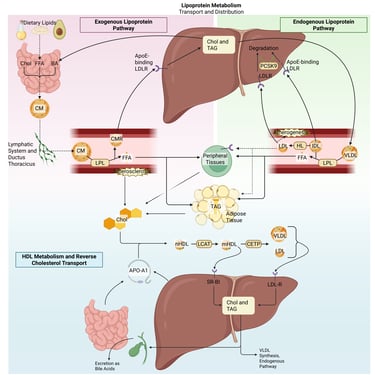
Detailed Illustration of the lipoprotein metabolism in the human body. Chol: Cholesterol, CM: Chylomicrons, CMR: Chylomicrone Remnants, TAG: Triacylglycerol, FFA: Free Fatty Acids, HDL: High Density lipoprotein, LDL: Low Density Lipoprotein, VLDL: Very Low Density Lipoprotein, LPL: Lipoprotein Lipase, HL: Hepatic Lipase, LCAT: Lecithin cholesterol acyltransferase, CETP: Cholesteryl ester transfer protein, LDLR: LDL-Receptor
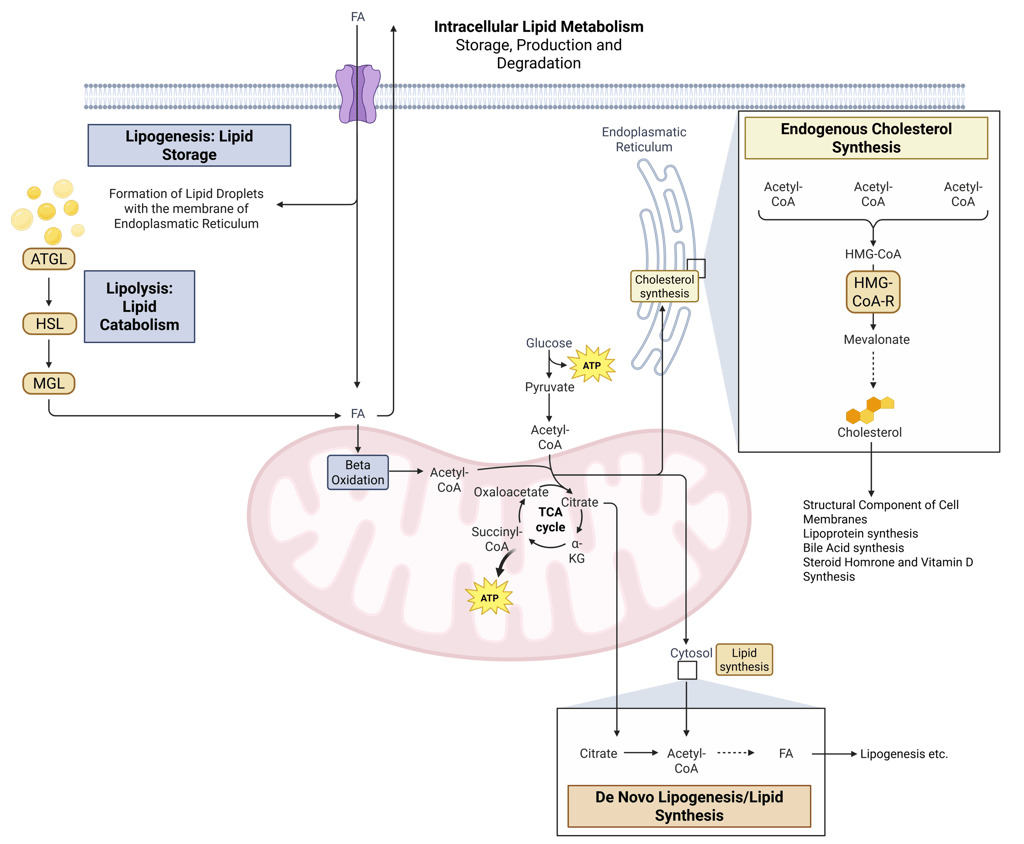
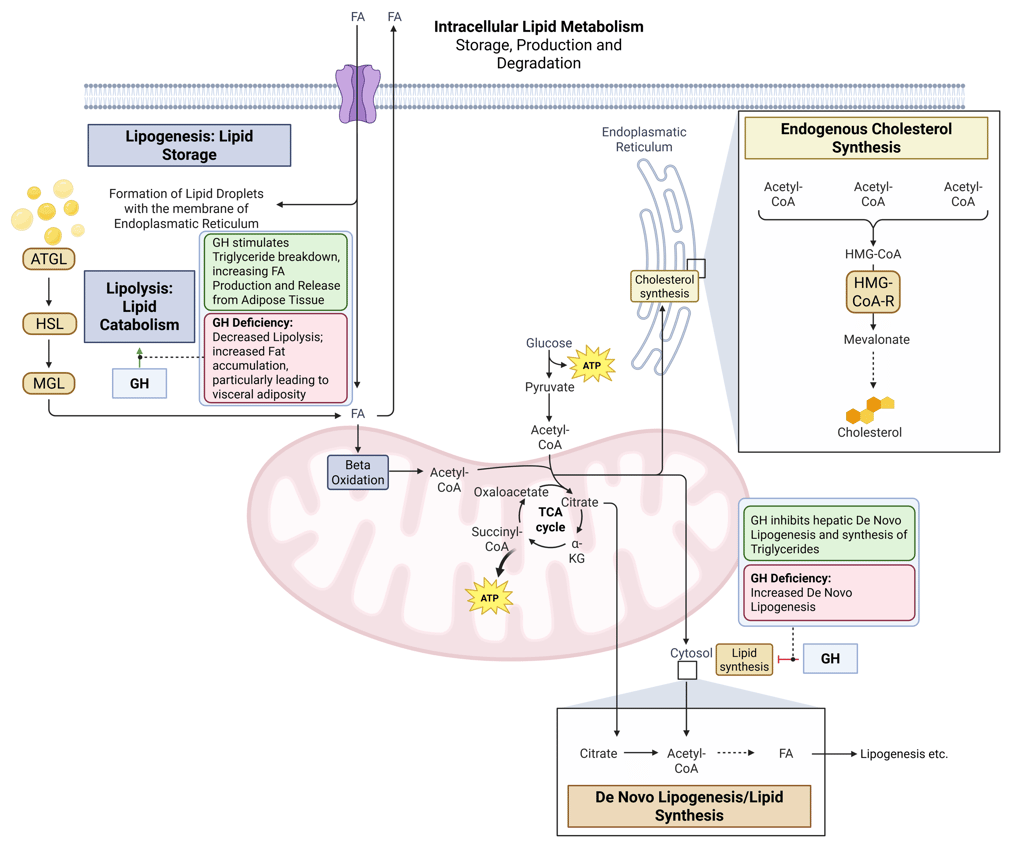
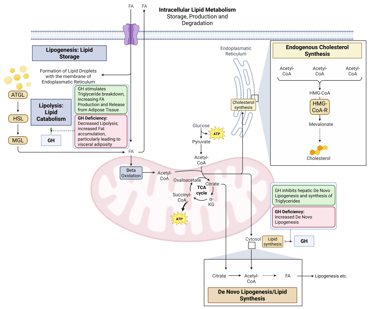
Detailed Illustration of intracellular Lipid Metabolism. HMG-CoA-R: HMG-CoA-Reductase, ATGL: Adipose Triglyceride Lipase, HSL: Hormone Sensitive Lipase, MGL: Monoglyceride Lipase, FA: FAtty Acids
The endocrine system exerts extensive control over both domains. Because of this, disturbances in hormonal signaling lead to characteristic changes in circulating lipids. Although considerable overlap exists across endocrine disorders, lipid profiles can still offer meaningful clues to an underlying hormonal imbalance. To illustrate this, consider the following three representative lipid patterns. Try to match each one to the most likely endocrine dysfunction.
Insulin Deficiency/Resistance
Hypothyroidism
GH-Deficiency
Before revealing the answers, we will outline the physiological and pathophysiological basis by which these endocrine disorders alter lipid homeostasis. Insulin, growth hormone, and thyroid hormones each regulate lipid metabolism at distinct and complementary control points, and their deficiency or impaired signaling produces predictable dyslipidemic patterns.
Insulin
Effects on intracellular lipid metabolism:
Insulin plays a central role in promoting lipid storage and suppressing lipid mobilization. It stimulates fatty-acid synthesis in the liver and adipose tissue by activating key enzymes and enhances the transcription of lipogenic genes. At the same time, insulin inhibits hormone-sensitive lipase, thereby reducing the release of free fatty acids (FFAs) from adipose tissue.
Pathophysiology: Insulin deficiency or impaired signaling leads to a loss of this anti-lipolytic effect, increasing adipose tissue lipolysis and raising FFA flux to the liver. This altered substrate availability subsequently impacts lipoprotein metabolism, as discussed below.
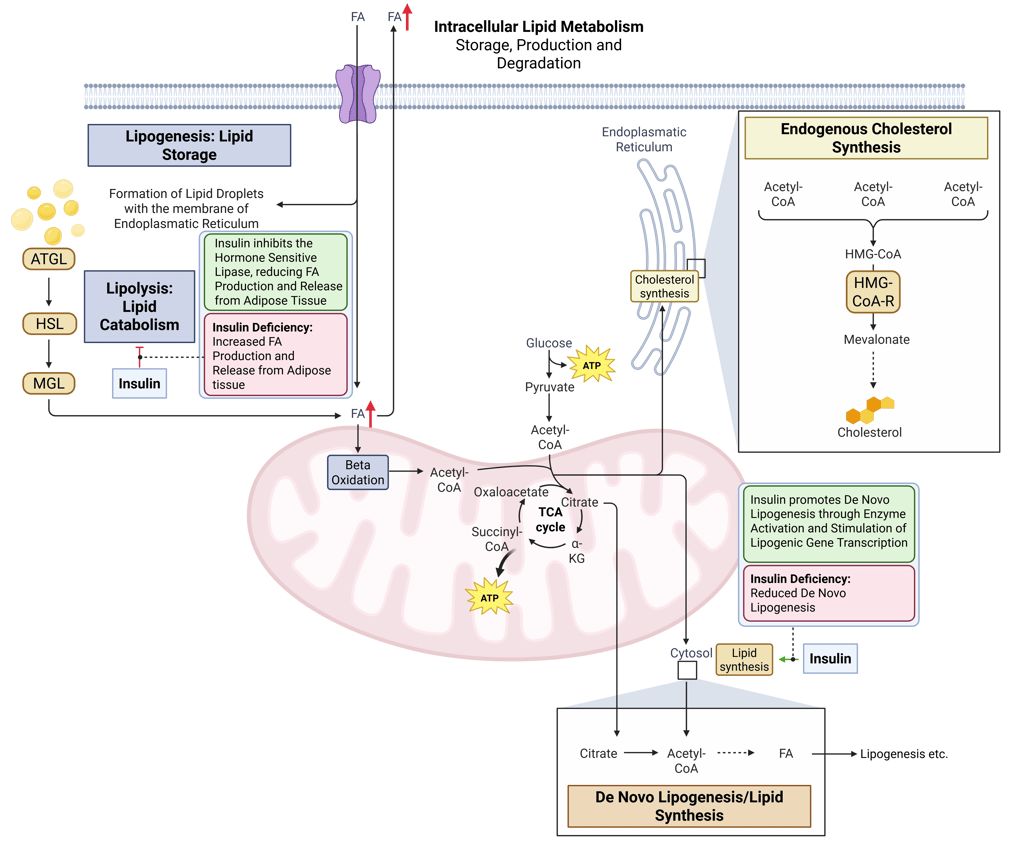
Physiological and pathophysiological effects of insulin and Insulin deficiency on intracellular Lipid Metabolism. HMG-CoA-R: HMG-CoA-Reductase, ATGL: Adipose Triglyceride Lipase, HSL: Hormone Sensitive Lipase, MGL: Monoglyceride Lipase, FA: FAtty Acids
Effects on lipoprotein metabolism:
FFAs delivered to the liver are used for VLDL synthesis and secretion. In insulin deficiency, the increased FFA supply drives excessive VLDL production, manifesting as hypertriglyceridemia.
In addition, insulin activity normally enhances lipoprotein lipase (LPL) function in peripheral tissues, promoting the clearance of triglyceride-rich lipoproteins such as VLDL and chylomicrons. In deficiency states, LPL activity is reduced, further contributing to accumulation of VLDL and chylomicrons, exacerbating hypertriglyceridemia.
The combination of increased production and decreased clearance results in an accumulation of triglyceride-rich particles and promotes the formation of small, dense LDL, which are particularly atherogenic.
Insulin also broadly regulates lipoprotein composition by supporting hepatic LDL receptor activity and promoting HDL formation. Consequently, insulin deficiency reduces LDL clearance and lowers HDL cholesterol levels..
This constellation of elevated triglycerides, low HDL cholesterol, and small dense LDL particles (may result in mild increase of LDL-Cholesterol) forms the characteristic dyslipidemic pattern of insulin deficiency, insulin resistance, and hyperglycemia.
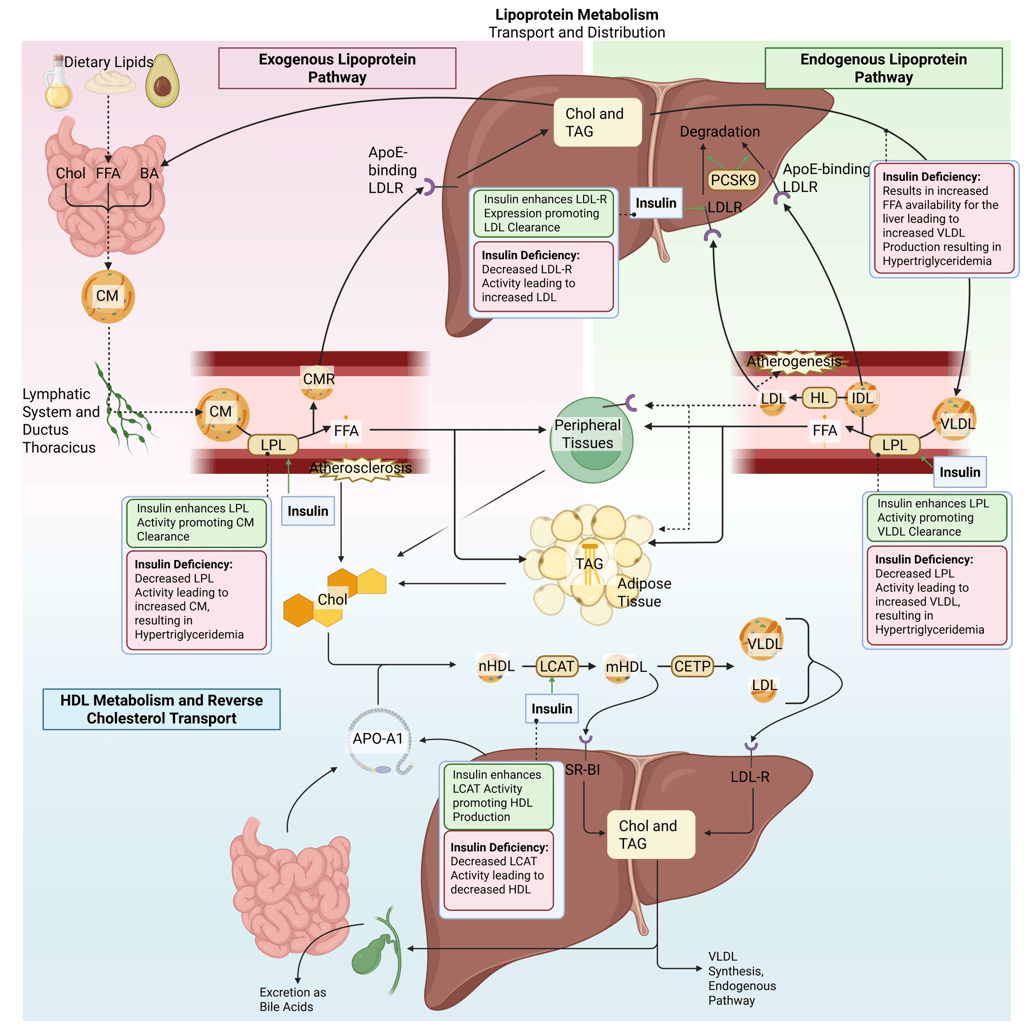
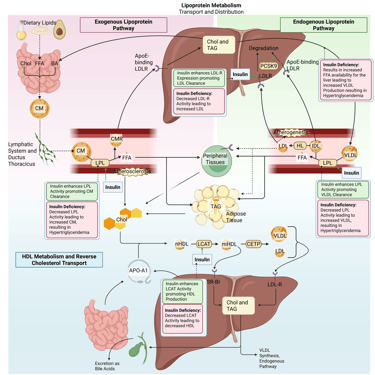
Physiological and pathophysiological effects of insulin and Insulin deficiency on the lipoprotein metabolism in the human body. Chol: Cholesterol, CM: Chylomicrons, CMR: Chylomicrone Remnants, TAG: Triacylglycerol, FFA: Free Fatty Acids, HDL: High Density lipoprotein, LDL: Low Density Lipoprotein, VLDL: Very Low Density Lipoprotein, LPL: Lipoprotein Lipase, HL: Hepatic Lipase, LCAT: Lecithin cholesterol acyltransferase, CETP: Cholesteryl ester transfer protein, LDLR: LDL-Receptor
Growth Hormone
Effects on Intracellular Lipid Metabolism
GH exerts primarily lipolytic effects in adipose tissue, stimulating triglyceride breakdown and increasing circulating FFAs. GH antagonizes insulin’s anti-lipolytic action, promoting fatty-acid flux and potentially contributing to insulin resistance. In the liver, GH modulates the expression of lipogenic enzymes and influences triglyceride secretion. Insulin can partially attenuate GH-mediated stimulation of hepatic lipogenesis and triglyceride production.
Pathophysiology: GH deficiency reduces these lipolytic and metabolic effects, leading to increased fat accumulation, particularly visceral adiposity and hepatic steatosis.
Physiological and pathophysiological effects of GH and GH deficiency on intracellular Lipid Metabolism. HMG-CoA-R: HMG-CoA-Reductase, ATGL: Adipose Triglyceride Lipase, HSL: Hormone Sensitive Lipase, MGL: Monoglyceride Lipase, FA: FAtty Acids
Effects on Lipoprotein Metabolism
GH normally upregulates hepatic LDL receptor expression and reduces PCSK9, enhancing LDL clearance. This also facilitates the hepatic uptake and catabolism of VLDL and its apolipoprotein B-100 (apoB-100) component, leading to increased VLDL clearance.
Pathophysology: In GH deficiency, reduced LDL receptor activity leads to higher circulating LDL and VLDL cholesterol. The latter is the main reason for hypertriglyceridemia in GH deficiency.
Changes in body composition, specifically increased visceral fat and reduced lean mass, further worsen dyslipidemia.
The net effect in GH deficiency is an increase in LDL-C, VLDL, and triglycerides, sometimes accompanied by reduced HDL cholesterol.
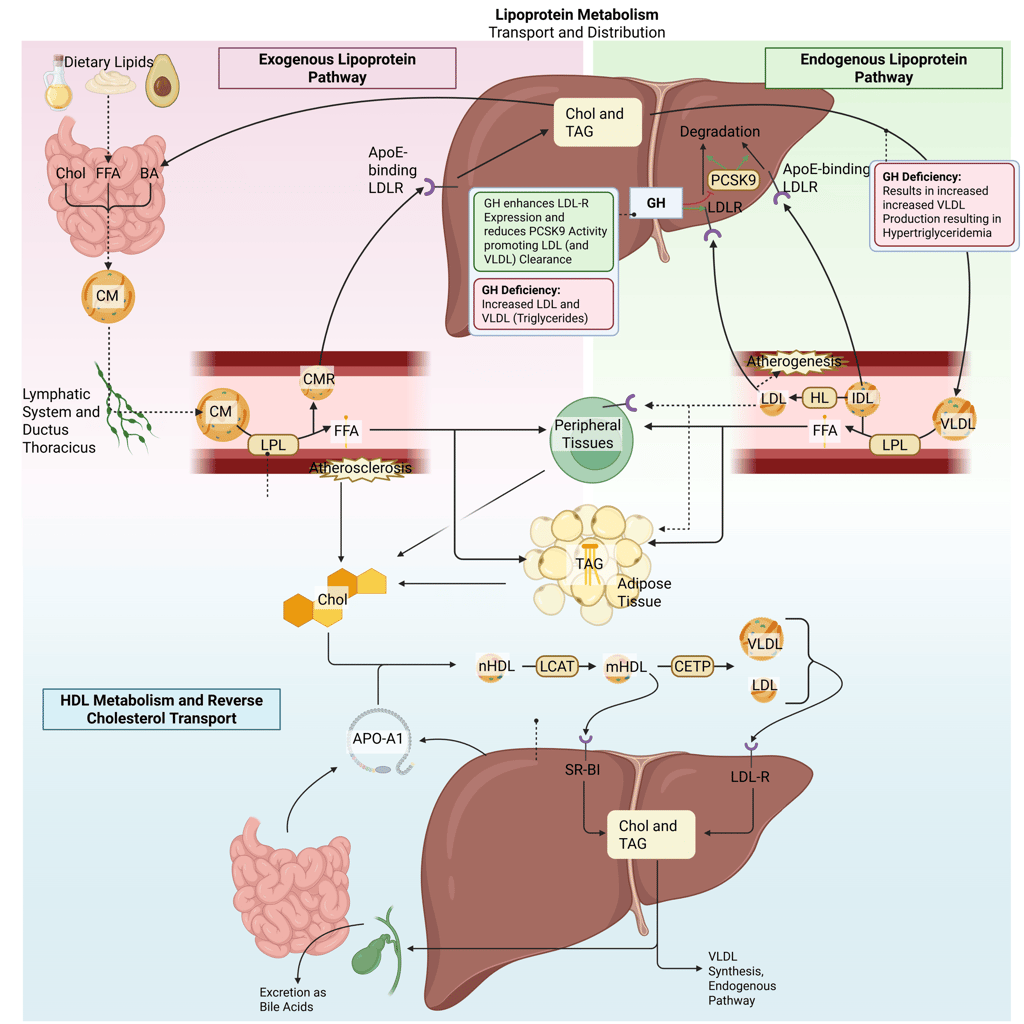
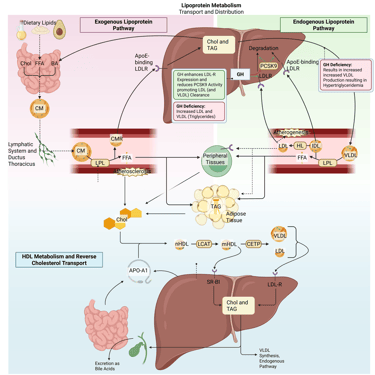
Physiological and pathophysiological effects of GH and GH deficiency on the lipoprotein metabolism in the human body. Chol: Cholesterol, CM: Chylomicrons, CMR: Chylomicrone Remnants, TAG: Triacylglycerol, FFA: Free Fatty Acids, HDL: High Density lipoprotein, LDL: Low Density Lipoprotein, VLDL: Very Low Density Lipoprotein, LPL: Lipoprotein Lipase, HL: Hepatic Lipase, LCAT: Lecithin cholesterol acyltransferase, CETP: Cholesteryl ester transfer protein, LDLR: LDL-Receptor
Thyroid Hormones
Effects on Intracellular Lipid Metabolism
Thyroid hormones regulate both lipid synthesis and degradation. They increase lipolysis and fatty-acid β-oxidation, while modulating de novo lipogenesis through transcriptional control of enzymes and regulators.
Pathophysiology: Hypothyroidism reduces these effects, leading to lower fatty-acid oxidation, decreased triglyceride hydrolysis, and impaired intracellular lipid turnover resulting in reduced lipolysis and increased lipid accumulation.
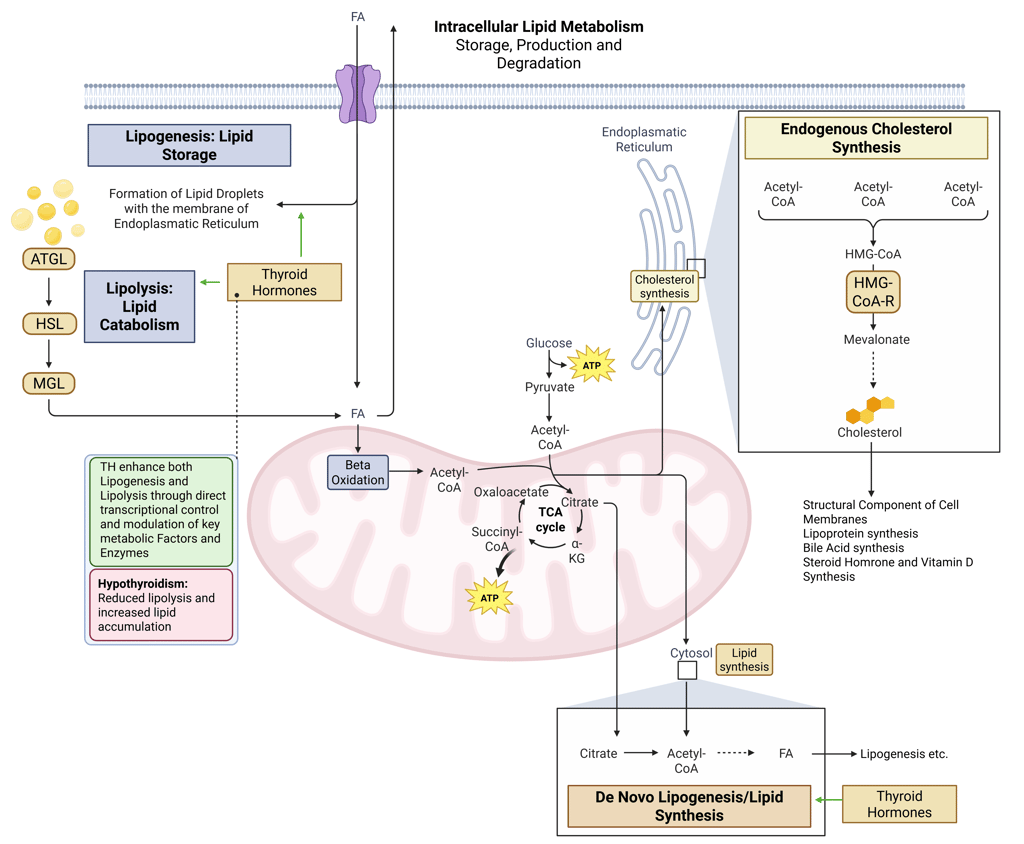
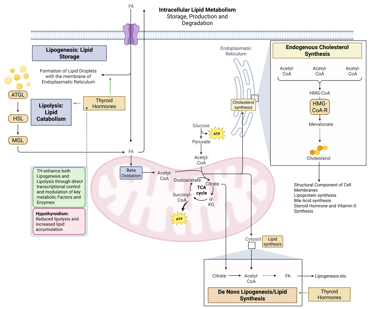
Physiological and pathophysiological effects of TH and Hypothyroidism on intracellular Lipid Metabolism. HMG-CoA-R: HMG-CoA-Reductase, ATGL: Adipose Triglyceride Lipase, HSL: Hormone Sensitive Lipase, MGL: Monoglyceride Lipase, FA: FAtty Acids
Effects on Lipoprotein Metabolism
Thyroid hormones enhance hepatic LDL receptor expression, increasing LDL clearance, and stimulate cholesterol 7α-hydroxylase, promoting bile acid synthesis. Thyroid hormones increase Lipoprotein Lipase (LPL) activity, promoting VLDL Clearance. They also promote HDL production by maintaining Cholesteryl ester transfer protein (CETP) function.
Pathophysiology: Hypothyroidism reduces LDL receptor activity and bile acid production, resulting in elevated LDL-C and total cholesterol.
Decreased Lipoprotein lipase activity impaires clearance of triglyceride-rich lipoproteins leading to moderate hypertriglyceridemia. Patients with hypothyroidism may have variable, often low HDL cholesterol levels.
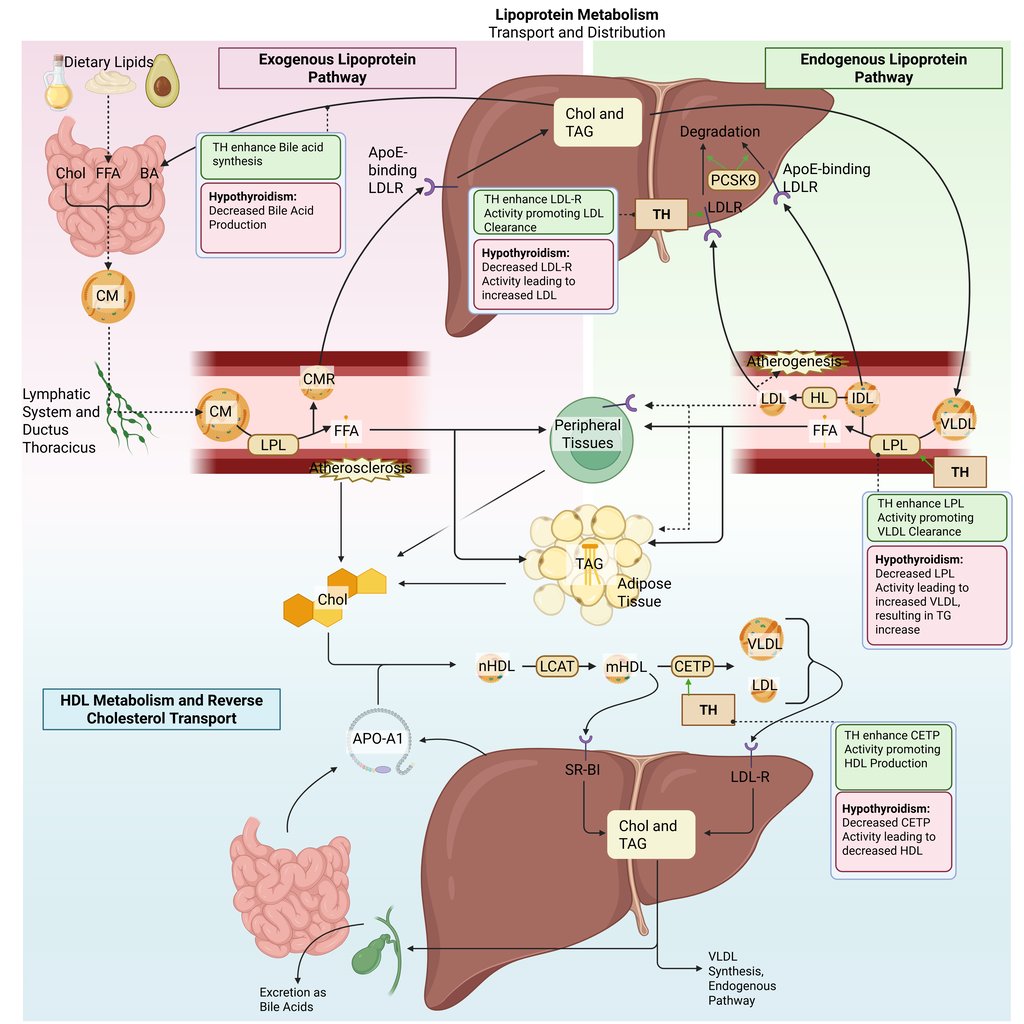
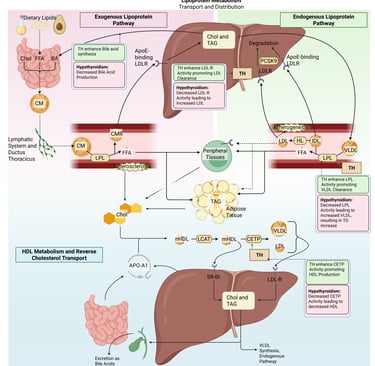
Physiological and pathophysiological effects of TH and Hypothyroidism on the lipoprotein metabolism in the human body. Chol: Cholesterol, CM: Chylomicrons, CMR: Chylomicrone Remnants, TAG: Triacylglycerol, FFA: Free Fatty Acids, HDL: High Density lipoprotein, LDL: Low Density Lipoprotein, VLDL: Very Low Density Lipoprotein, LPL: Lipoprotein Lipase, HL: Hepatic Lipase, LCAT: Lecithin cholesterol acyltransferase, CETP: Cholesteryl ester transfer protein, LDLR: LDL-Receptor
In summary, Insulin Deficiency/Resistance, GH Deficiency and Hypothyroidism therefore typically lead to the following lipid profiles:
References
All Illustrations were created in https://BioRender.com
Damiano, Fabrizio, Alessio Rochira, Antonio Gnoni, und Luisa Siculella. 2017. „Action of Thyroid Hormones, T3 and T2, on Hepatic Fatty Acids: Differences in Metabolic Effects and Molecular Mechanisms“. International Journal of Molecular Sciences 18 (4): 744. https://doi.org/10.3390/ijms18040744.
Duntas, Leonidas H., und Gabriela Brenta. 2018. „A Renewed Focus on the Association Between Thyroid Hormones and Lipid Metabolism“. Frontiers in Endocrinology 9: 511. https://doi.org/10.3389/fendo.2018.00511.
Frick, Fredrik, Daniel Lindén, Caroline Améen, Staffan Edén, Agneta Mode, und Jan Oscarsson. 2002. „Interaction between Growth Hormone and Insulin in the Regulation of Lipoprotein Metabolism in the Rat“. American Journal of Physiology. Endocrinology and Metabolism 283 (5): E1023-1031. https://doi.org/10.1152/ajpendo.00260.2002.
Hepprich, Matthias, Fahim Ebrahimi, und Emanuel Christ. 2023. „Dyslipidaemia and Growth Hormone Deficiency - A Comprehensive Review“. Best Practice & Research. Clinical Endocrinology & Metabolism 37 (6): 101821. https://doi.org/10.1016/j.beem.2023.101821.
Hirano, Tsutomu. 2018. „Pathophysiology of Diabetic Dyslipidemia“. Journal of Atherosclerosis and Thrombosis 25 (9): 771–82. https://doi.org/10.5551/jat.RV17023.
Howard, B. V. 1999. „Insulin Resistance and Lipid Metabolism“. The American Journal of Cardiology 84 (1A): 28J-32J. https://doi.org/10.1016/s0002-9149(99)00355-0.
Jonklaas, Jacqueline. 2024. „Hypothyroidism, lipids, and lipidomics“. Endocrine 84 (2): 293–300. https://doi.org/10.1007/s12020-023-03420-9.
Kotwal, Anupam, Tiffany Cortes, Natalia Genere, u. a. 2020. „Treatment of Thyroid Dysfunction and Serum Lipids: A Systematic Review and Meta-Analysis“. The Journal of Clinical Endocrinology and Metabolism 105 (12): dgaa672. https://doi.org/10.1210/clinem/dgaa672.
Krauss, Ronald M. 2004. „Lipids and Lipoproteins in Patients with Type 2 Diabetes“. Diabetes Care 27 (6): 1496–504. https://doi.org/10.2337/diacare.27.6.1496.
Liu, Zhongbo, Jose Cordoba-Chacon, Rhonda D. Kineman, u. a. 2016. „Growth Hormone Control of Hepatic Lipid Metabolism“. Diabetes 65 (12): 3598–609. https://doi.org/10.2337/db16-0649.
Newman, Connie B, Michael J Blaha, Jeffrey B Boord, u. a. 2020. „Lipid Management in Patients with Endocrine Disorders: An Endocrine Society Clinical Practice Guideline“. The Journal of Clinical Endocrinology & Metabolism 105 (12): 3613–82. https://doi.org/10.1210/clinem/dgaa674.
Ritter, Megan J., Izuki Amano, und Anthony N. Hollenberg. 2020. „Thyroid Hormone Signaling and the Liver“. Hepatology (Baltimore, Md.) 72 (2): 742–52. https://doi.org/10.1002/hep.31296.
Sharma, Rita, John J. Kopchick, Vishwajeet Puri, und Vishva M. Sharma. 2020. „Effect of Growth Hormone on Insulin Signaling“. Molecular and Cellular Endocrinology 518 (Dezember): 111038. https://doi.org/10.1016/j.mce.2020.111038.
Sinha, Rohit A., Brijesh K. Singh, und Paul M. Yen. 2018. „Direct Effects of Thyroid Hormones on Hepatic Lipid Metabolism“. Nature Reviews. Endocrinology 14 (5): 259–69. https://doi.org/10.1038/nrendo.2018.10.
Zhang, Dengke, Yanghui Wei, Qingnan Huang, u. a. 2022. „Important Hormones Regulating Lipid Metabolism“. Molecules (Basel, Switzerland) 27 (20): 7052. https://doi.org/10.3390/molecules27207052.
© 2025 EndoCases. All rights reserved.
This platform is intended for medical professionals, particularly endocrinology residents, and is provided for educational purposes only. It supports learning and clinical reasoning but is not a substitute for professional medical advice or patient care. The information is general in nature and should be applied with appropriate clinical judgment and in accordance with local guidelines.
Portions of the text on this website were edited with the assistance of Artificial Intelligence to improve grammar and phrasing, as English is not my first language. All medical content, ideas for illustrations, and overall structure are original and based on the author’s own expertise and the cited medical literature. No AI tools were used to generate or influence the educational content itself.
All of the content is independent of my employer.
Use of this site implies acceptance of our Terms of Use
Contact us via E-Mail: contact@endo-cases.com