Laboratory Measurement in Clinical Endocrinology
The fact that hormones are transported through the bloodstream provides a valuable opportunity for assessing endocrine function. In this sense, the circulatory system serves as an indirect window into endocrine activity. Measuring hormone concentrations in blood is a cornerstone of endocrine diagnostics. Therefore, it is important to understand some fundamental principles underlying laboratory hormone measurements.
In clinical endocrinology, laboratory testing typically includes the measurement of hormones themselves, binding proteins, and substances under hormonal control, which serve as surrogate markers of hormone action. A classic example is the evaluation of insulin action, where blood glucose levels are used as an indirect indicator of insulin activity. Similarly, in calcium–phosphate homeostasis, a combination of serum calcium, phosphate, and parathyroid hormone (PTH) measurements is used to assess endocrine regulation.
However, in other endocrine axes—such as the hypothalamic–pituitary–thyroid axis—there is no direct, easily measurable blood marker that reflects peripheral hormone action. In such systems, the measurement of circulating hormone concentrations becomes a key tool in for evaluating endocrine function. While this is only possible because hormones are present in the bloodstream, interpreting hormone levels as direct indicators of functional status has its limitations. Since measuring hormone concentrations in the blood is fundamental to endocrine diagnostics, it is crucial to understand the underlying principles and potential limitations of laboratory hormone measurements. These aspects will be explored in the following paragraphs.
General Principles of Hormone Measurements
Laboratory evaluation in endocrinology typically follows a structured sequence, beginning with a screening test—most often a timed measurement of basal hormone levels—and proceeding, if needed, to confirmation through dynamic testing.
Static Testing and Timing
Initial hormone measurements are commonly taken at specific times to align with known physiological secretion patterns. For example, cortisol is best assessed in the early morning when levels peak, particularly when investigating adrenal insufficiency. Conversely, late evening cortisol may be used to assess suspected hypercortisolism, as levels should be low at that time.
Random hormone measurements can be misleading due to the pulsatile nature of many endocrine secretions, potentially capturing either peaks or troughs. Therefore, the clinical utility of random samples depends on the hormone’s secretion stability and the standardization of preanalytical conditions, including fasting status, stress, age, and sex.
Hormones such as thyroid hormones, prolactin (PRL), and insulin-like growth factor 1 (IGF-1) tend to have more stable circulating levels and can often be accurately measured in fasting morning serum samples, depending on the clinical context.
In contrast, hormones with marked episodic or circadian variation (like cortisol or Gonadotropins, Estrogen and Progesterone in premenopausal females) require carefully timed samples for accurate interpretation.
Dynamic Function Testing
If initial testing indicates potential endocrine dysfunction, dynamic tests are used for confirmation by either stimulating or suppressing hormone secretion:
Hormone Concentrations
The concentration of a hormone in the bloodstream is determined by a combination of its secretory dynamics, metabolic processing, and clearance rates. While the mechanisms of metabolism and clearance will be discussed in detail later, it is important to note that hormones circulate at widely varying concentrations. Due to significant dilution following secretion, the bioactive forms of many hormones are often present at relatively low levels—frequently in the picomolar range—as illustrated below.
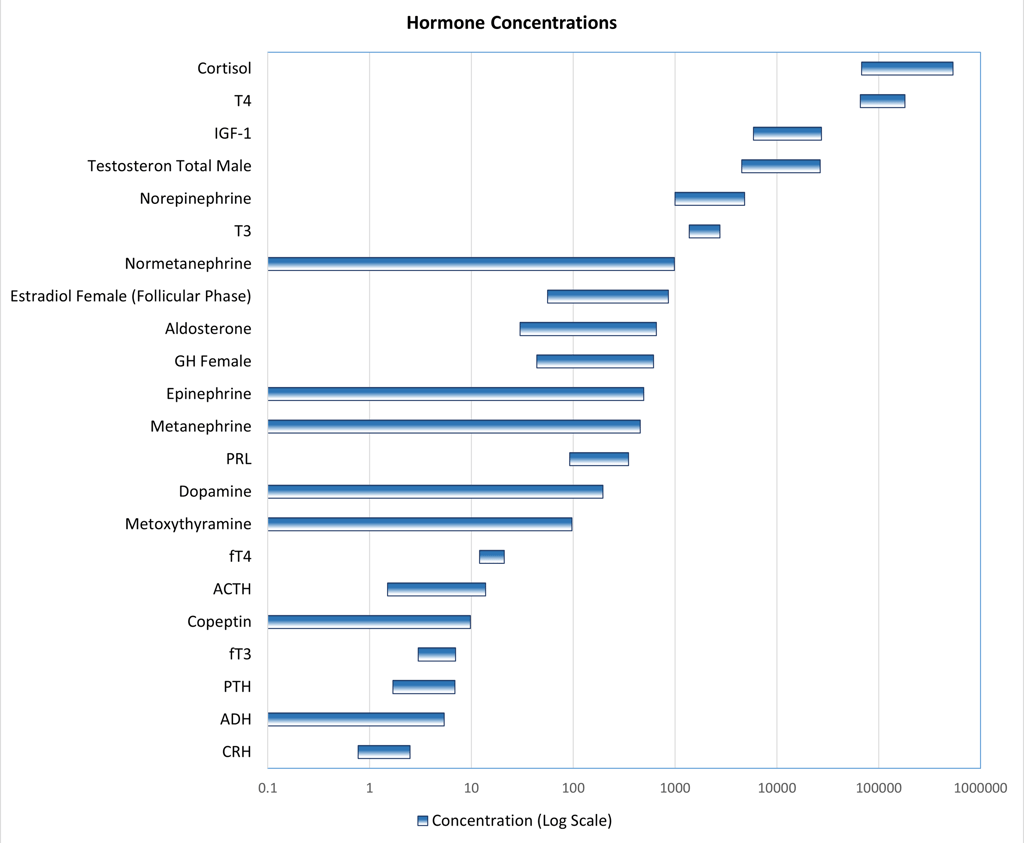
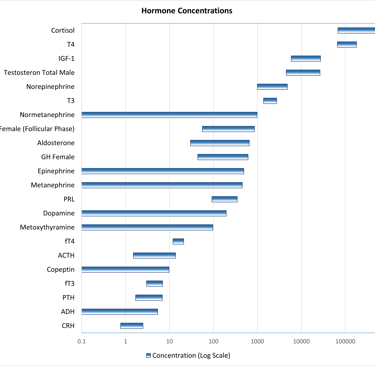
Graphic: Reference ranges of different hormones and metabolites used in laboratory department of a a tertiary hospital in switzerland on a Log10-scale. Inspired by:
Patrick M. Sluss, Frances J. Hayes, Chapter 6 - Laboratory Techniques for Recognition of Endocrine Disorders, Editor(s): Shlomo Melmed, Kenneth S. Polonsky, P. Reed Larsen, Henry M. Kronenberg, Williams Textbook of Endocrinology (Thirteenth Edition), Elsevier, 2016, Pages 77-107, ISBN 9780323297387, https://doi.org/10.1016/B978-0-323-29738-7.00006-X.(https://www.sciencedirect.com/science/article/pii/B978032329738700006X)
Hormone Concentrations as Indicators of Endocrine Function
In clinical practice, the ideal scenario is that the concentration of a hormone in the bloodstream accurately reflects its secretion and biological activity. This requires several conditions:
The hormone must be present at a concentration within the detectable range of available assays
The hormone should have sufficient stability in circulation; its secretion rate should be relatively constant or follow a predictable circadian rhythm
Validated, accessible, and precise methods of detection must be available
Ideally, the assay should measure the bioactive form—often the free (unbound) hormone fraction.
However, in reality, these conditions are not always met. The accurate interpretation of hormone levels thus relies heavily on clinical context and the expertise of the clinician. Laboratory results may be influenced by suboptimal testing conditions (e.g., during critical illness), or by methodological limitations such as assay interference, particularly in immunoassays (discussed further below). In some cases, total hormone concentrations are measured—for example, total testosterone is commonly used to assess gonadal function in men—even though this may not fully represent biological activity.
Moreover, hormonal concentrations in the blood do not always correlate with physiological action. The body possesses complex regulatory mechanisms—such as receptor desensitization or downregulation—that modulate hormonal effects independently of circulating levels. For instance, catecholamine receptor desensitization can blunt physiological responses despite normal or elevated hormone levels. Additionally, some hormones exert their effects primarily through intracellular activation, meaning that plasma concentrations may not reflect their true biological potency or activity.
Available Specimens for Hormone Measurements
Many specimen types are used to measure analytes in bodily fluids, but in clinical endocrinology, certain specimens are preferred for hormone measurement.
Whole blood offers the advantage of detecting rapid changes in response to stimuli, which is valuable for tracking dynamic hormone fluctuations. It is particularly useful for analyzing labile analytes, as it can be collected and tested quickly, often at the point of care, improving cost-effectiveness and patient management. Collection via dried blood spots on filter paper (from finger or heel punctures) provides a convenient method for transport and hormone measurement, with reliable correlation to serum levels when standardized procedures are followed.
However, whole blood use is limited by several factors:
The need to prevent clotting requires anticoagulants, which may interfere with assays.
Its complex composition, including cellular components, can interfere with analytic methods.
To address these challenges, whole blood specimens are often diluted (requiring either high analyte concentrations or highly sensitive assays) or preprocessed to remove cells or reduce complexity.
Serum is obtained by allowing whole blood to clot, typically in glass tubes, followed by centrifugation to separate the serum from the clot. This process yields a cell-free specimen, with many clotting proteins also removed.
In some laboratories, glass tubes have been replaced by safer plastic tubes to reduce breakage, especially in fully automated settings. However, whole blood does not clot efficiently in plastic tubes, necessitating the use of clot activators or enhancers. These additives can interfere with analytical methods, requiring thorough validation to ensure assay accuracy.
Plasma is obtained by preventing clotting in whole blood followed by centrifugation to separate the cellular components. Common anticoagulants include ethylenediaminetetra-acetic acid (EDTA), citrate, and heparin. EDTA, in particular, also inhibits proteolysis, making it advantageous for measuring labile analytes such as adrenocorticotropic hormone (ACTH) or parathyroid hormone (PTH).
Additional additives may be included for specific tests, sodium fluoride for example, is added to EDTA tubes to inhibit glycolysis for accurate glucose measurements. However, these additives can interfere with certain assays, necessitating careful validation.
Urine: Urine samples can contain both the original hormones and their metabolites, which may have varying biological activity. The 24-hour urine collection is frequently used in endocrine testing because it integrates an average hormone level over time, capturing the fluctuations caused by pulsatile secretion throughout the day. This method also enhances the detection sensitivity for certain hormones and their metabolites.
However, the collection process can be inconvenient and subject to delays. There is also uncertainty regarding whether the entire urine volume has been collected. To help assess the completeness of the sample, urinary creatinine levels are measured and compared to the individual's muscle mass. Since many hormones in urine are bound to carrier proteins before excretion, liver function, and to a lesser degree kidney function, can affect the hormone levels measured in urine.
Saliva is an appealing alternative specimen for measuring non–protein-bound hormones and small molecules. Small analytes from blood enter saliva by crossing capillary walls, basement membranes, and lipophilic epithelial cell membranes through processes such as passive diffusion, ultrafiltration, and active transport. The concentration of these analytes in saliva depends on factors like their free (non–protein-bound) levels in blood, salivary pH, the analyte’s acid dissociation constant (pKa), and molecular size. Typically, molecules smaller than 500 Da that are nonionized and not bound to proteins pass into saliva by passive diffusion. As with any sample type, saliva must undergo thorough validation for each analyte and assay to establish accurate reference ranges and ensure reliable results.
Laboratory Techniques of Hormone measurements
In clinical endocrinology, hormones can be measured using a variety of laboratory techniques, each suited to specific clinical and biochemical needs. The choice of method depends on factors such as the hormone's biochemical form (e.g., free or bound), its concentration in the bloodstream, and the clinical context. Additionally, considerations like the cost, availability, and sensitivity of the test play a significant role in determining the appropriate technique. Common methods include immunoassays, chromatographic techniques and mass spectrometry, each offering distinct advantages based on the nature of the hormone and the clinical question at hand.
Laboratory techniques for hormone measurement can be broadly categorized into two main groups: antibody-based methods (such as immunoassays) and molecular structure–based methods (such as mass spectrometry and chromatography).
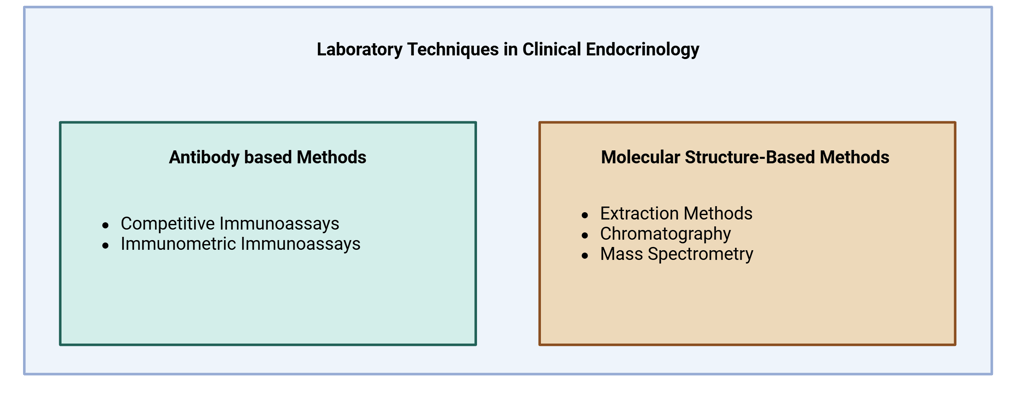
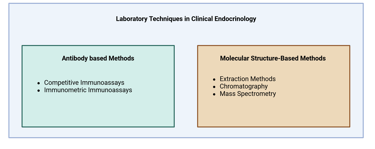
Illustration Main Laboratory Techniques used in Clinical Endocrinology
Antibody based methods
The fundamental principle of antibody-based methods is the use of an antibody that specifically binds to the analyte of interest, combined with either a labeled antigen or a labeled antibody. This interaction generates a measurable signal, which can be correlated to the concentration of the analyte. There are two main methodological approaches within this group: competitive assays, where the analyte competes with a labeled version for antibody binding, and immunometric (or sandwich) assays, which use two antibodies to capture and detect the analyte. This sensitive technique has allowed ultrasensitive measurements of physiologic hormone concentrations. These two methods are discussed in further detail below. Following are the basic requirements and components of Antibody-Based Hormone Assays.
Analyte: The hormone of interest to be quantified.
Antibody: Specific to the analyte—ideally with high affinity to ensure strong specificity and minimal cross-reactivity. Both polyclonal and monoclonal antibodies are used:
Polyclonal antibodies recognize multiple epitopes on the analyte, providing broader detection and greater tolerance to analyte variability. However, they have limitations in batch-to-batch consistency and are less amenable to standardization.
Monoclonal antibodies bind to a single epitope with high specificity, reproducibility, and scalability. Their narrow recognition can, however, limit detection of certain biologically active hormone variants.
Signal generation: A detectable signal must be produced, usually through a labeled component:
Label for signal generation. These include radioactive, enzymatic, chemiluminescent and fluorescent labels
Labeled analyte, used in competitive immunoassays
Labeled antibody, used in immunometric (sandwich) assays
Separation system: A mechanism is required to separate bound from unbound components, which is essential for accurate quantification. This step may involve washing, centrifugation, or magnetic separation depending on the assay format.
Signal quantification: The generated signal (radioactive, enzymatic, chemiluminescent, or fluorescent) is measured using appropriate detection systems. The intensity of this signal is correlated to the concentration of the hormone depending on the method used.
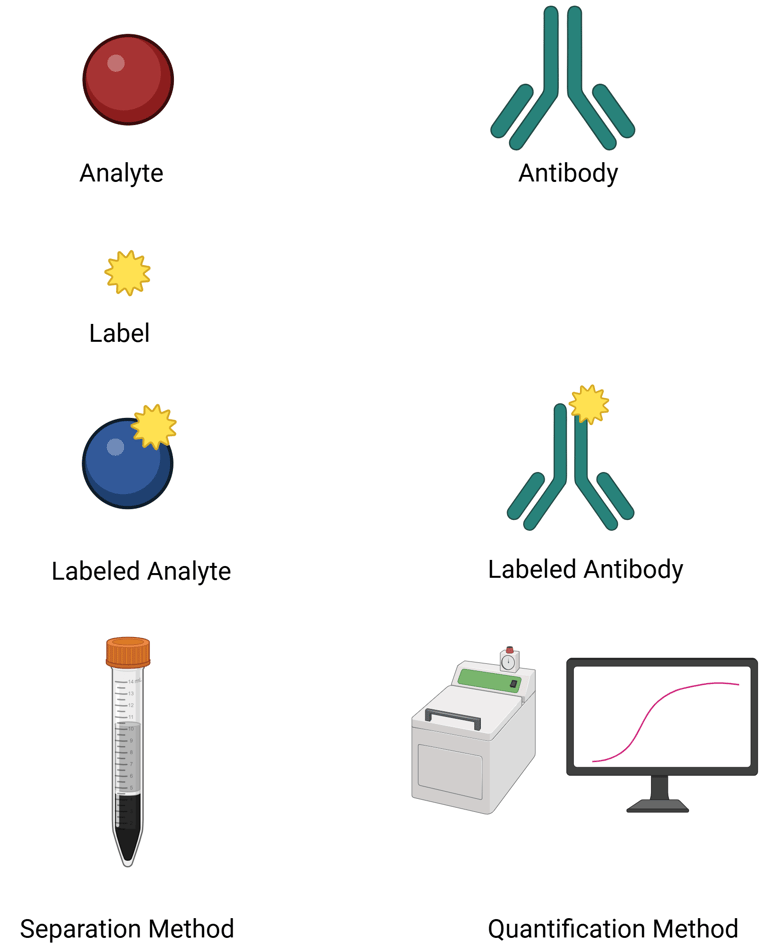
Illustration: Components of AB based Assays
The naming of immunoassays is typically based on the type of label used for signal detection:
Radioactive labels → used in Radioimmunoassay (RIA)
Enzymatic labels → used in ELISA and other enzyme immunoassays (EIA)
Chemiluminescent labels → used in chemiluminescent immunoassays (CLIA)
Fluorescent labels → used in fluoroimmunoassays (FIA)
In clinical endocrinology, chemiluminescent immunoassays (CLIA) and enzyme immunoassays (EIA) are the most commonly employed techniques for hormone measurement.
CLIA is widely favored for its high sensitivity, specificity, and rapid turnaround time. It is commonly used to measure hormones such as thyroid-stimulating hormone (TSH), free thyroxine (FT4), and intact parathyroid hormone (PTH). Additionally, CLIA is effective in measuring aldosterone and renin, which are essential in diagnosing conditions like primary aldosteronism.
EIA, including the well-known ELISA, remains a versatile and cost-effective method in clinical settings. It is routinely used for quantifying hormones such as luteinizing hormone (LH), follicle-stimulating hormone (FSH), and prolactin.
RIA, once considered the gold standard due to its excellent sensitivity and specificity, is now less commonly used because of safety concerns, regulatory restrictions, and the complexity of handling radioactive materials. Nonetheless, it still holds value in specialized laboratories and research, particularly for certain steroid hormone assays.
Classic Competitive Immunoassay
A competitive binding assay is a method used to quantify analytes—such as hormones—in which the analyte in a patient's sample competes with a labeled version of the same analyte for a limited number of antibody binding sites. This approach formed the basis of early clinical hormone assays, especially radioimmunoassays (RIA).
The three essential components are:
Antibody (specific to the analyte),
Labeled analyte (radioactive, enzymatic, or other detectable tag),
Unlabeled analyte (from the sample or calibrators).
The core principle of this methodology is to allow a steady-state or equilibrium condition to be established, where both the labeled and unlabeled analytes compete to bind with the limited antibody sites. This process follows the law of mass action, governed by the relative affinities and concentrations of the analytes.
When the concentrations of both the antibody and labeled analyte are held stable, the amount of labeled analyte that binds is inversely proportional to the concentration of unlabeled analyte in the sample. The more analyte present in the patient sample, the less labeled analyte is bound.
By comparing the proportion of bound labeled analyte (often expressed as % bound/total) against a standard curve derived from known analyte concentrations, the analyte level in the sample can be precisely determined.
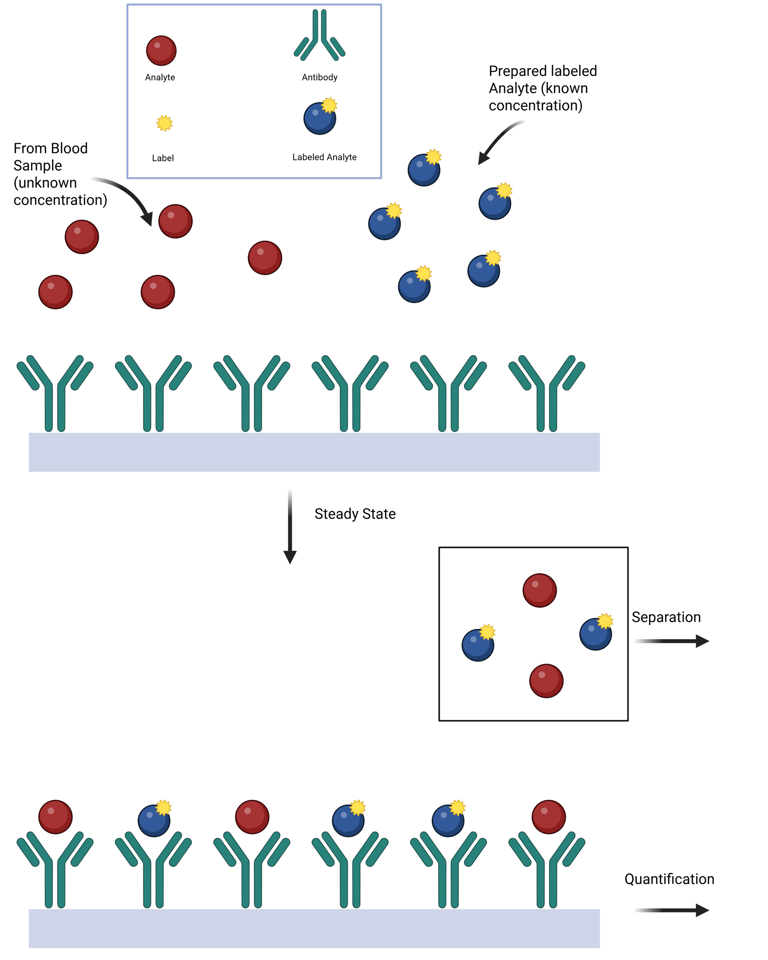
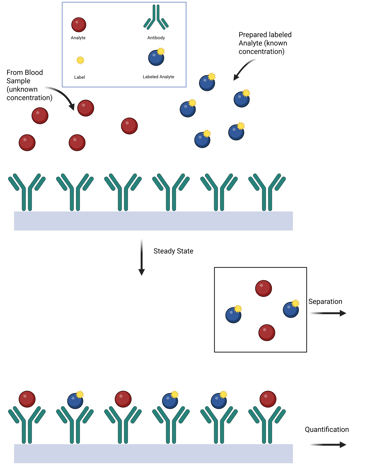
Illustration: Competitive Immuno-Assay
Epitope-Specific Immunometric Assays
For larger analytes containing multiple non-overlapping antigenic epitopes, the development of monoclonal antibodies has enabled the creation of assays that use two antibodies. In this approach, a solid-phase monoclonal antibody (the capture antibody) is specific to one epitope and is used to bind the antigen (analyte) in the sample. A second labeled monoclonal antibody (the detection antibody), which targets a different epitope, allows for the quantification of the captured antigen after unbound reagents are washed away. The signal measured is directly proportional to the hormone concentration.
Modern signaling systems, such as electrochemiluminescence, are commonly used in fully automated clinical immunoanalyzers. Unlike competitive immunoassays, these assays use a large excess of antibody-binding sites relative to the antigen concentration. They are termed immunometric assays because the binding reaction occurs rapidly (following first-order kinetics due to the excess of antibody), meaning it is unnecessary to establish a binding equilibrium (as required in competitive assays) before measuring the amount of label associated with the immune complex.
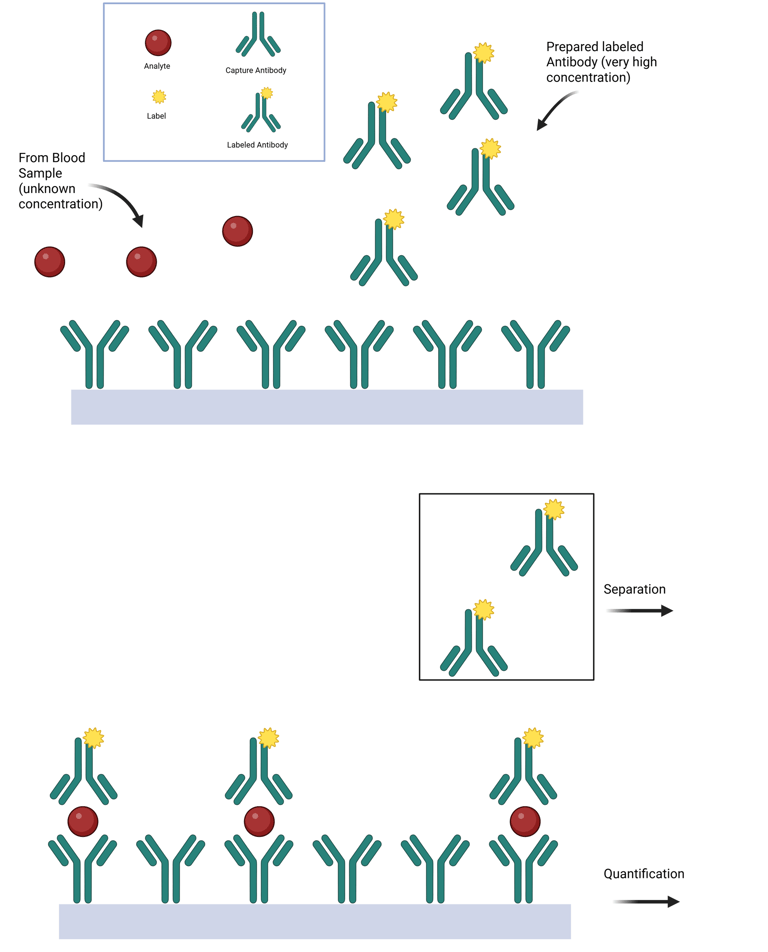
Illustration: Immunometric Assay
Limitations of antibody based hormone measurements
A key indicator of performance in antibody-based assays is analytical specificity—the ability of the assay to accurately measure only the intended analyte while excluding potential interferences.
The primary factor influencing analytical specificity is cross-reactivity, which refers to the assay's tendency to generate a signal in response to molecules structurally similar to the target analyte, without having the same biological function. Cross-reactivity is typically predictable and inherent to the antibody's binding characteristics. For instance, in steroid hormone assays, structurally related compounds may bind to the antibody and displace the labeled analyte, leading to an inaccurate signal that mimics the presence of the target hormone.
Another major source of error is interference, which differs from cross-reactivity in that it either alters the signal produced by the target analyte or generates a signal independently, without a predictable relationship to analyte concentration. Common sources of interference in clinical endocrinology include:
Specimen-related interference in signal detection, especially in assays relying on light or fluorescence. For example, hemolyzed, lipemic, or icteric samples may distort optical signals. Lipemia, in particular, can affect the accurate quantification of water-soluble analytes.
Analyte-specific interference, such as the degradation of protease-sensitive proteins in hemolyzed samples due to enzyme release, independent of optical signal distortion.
Immunometric assay-specific interferences, including:
The high-dose hook effect, where very high analyte concentrations saturate both capture and detection antibodies, leading to falsely low results (see below).
Heterophile antibody interference
Heterophile antibody interference, caused by nonspecific antibodies in the patient's sample that bridge the capture and detection antibodies, resulting in false-positive signals (see below).
Non-Heterophile Antibody Interference
The Hook Effect. The hook effect is a phenomenon in immunometric assays where extremely high concentrations of an analyte paradoxically result in falsely low measured values.
When antigen concentrations approach the binding capacity of the capture antibody, the assay signal plateaus, and no longer rises proportionally with antigen levels. Beyond this threshold, laboratories typically dilute the specimen to remain within the measurable range.
However, when the antigen concentration greatly exceeds the capture antibody’s capacity, excess antigen can saturate both the capture and detection antibodies, preventing the formation of the antibody–antigen–antibody "sandwich" complex and causing a paradoxical decrease in signal. In extreme cases, all available antibody binding sites may become occupied by free antigen, resulting in an undetectable signal that falsely suggests the absence of analyte. Recognizing this “high-dose hook effect” is essential, particularly when measuring hormone levels in patients with hormone-secreting tumors. This phenomenon has been well documented in assays for prolactin, hCG, thyroglobulin, calcitonin, and α-fetoprotein.
Modern assays have greatly reduced the occurrence of the hook effect through specific methodological improvements. These include:
Sample dilution, which brings analyte concentrations within the assay’s dynamic range;
High-capacity solid-phase antibodies, which increase the threshold at which the hook effect occurs; and
Two-step assay designs, where the capture and detection antibodies are applied sequentially to prevent premature saturation.
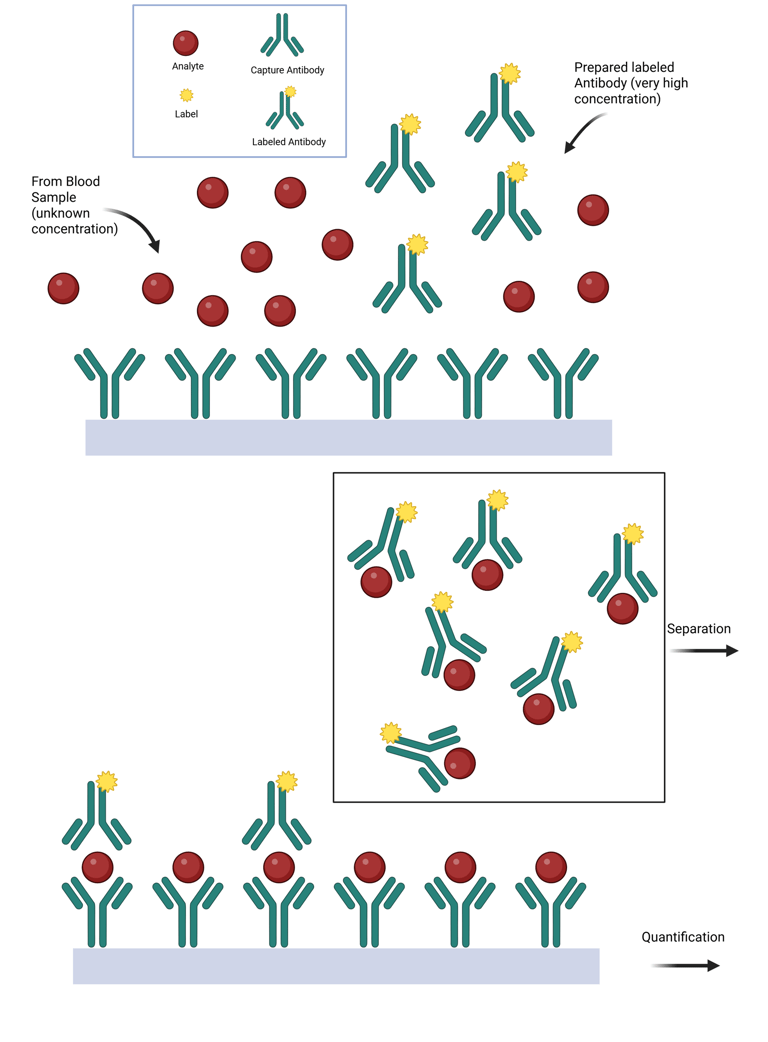
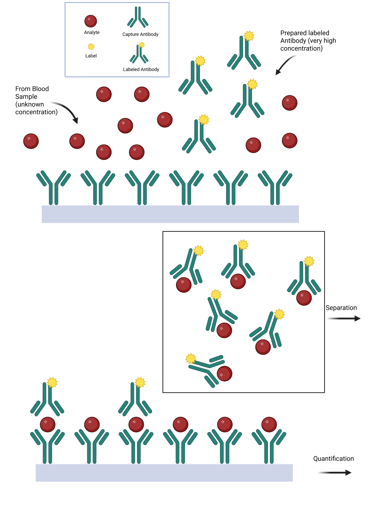
Illustration: Hook Effect
Heterophile antibody interference occurs when endogenous antibodies—typically with broad and nonspecific affinity for various antigens, including animal-derived immunoglobulins—disrupt immunoassay function. Due to their bivalent nature, these antibodies can inadvertently bridge the capture and detection antibodies in immunometric assays even in the absence of the target analyte, producing falsely elevated results.
Most individuals have only low titers of heterophile antibodies, and modern immunoassays include blocking agents to neutralize their effect. However, in certain individuals with high titers, such as patients exposed to therapeutic monoclonal antibodies or animal-derived immunoglobulins, these blockers may be insufficient, leading to spurious assay results. In some cases, the source of interference is identifiable; in others, it remains clinically unpredictable.
Use of Single-Chain Antibodies (scFv): Employing single-chain antibodies can reduce heterophilic antibody interference, which can contribute to the hook effect. Single-chain antibodies have a smaller size and reduced likelihood of cross-linking, thereby improving assay reliability.
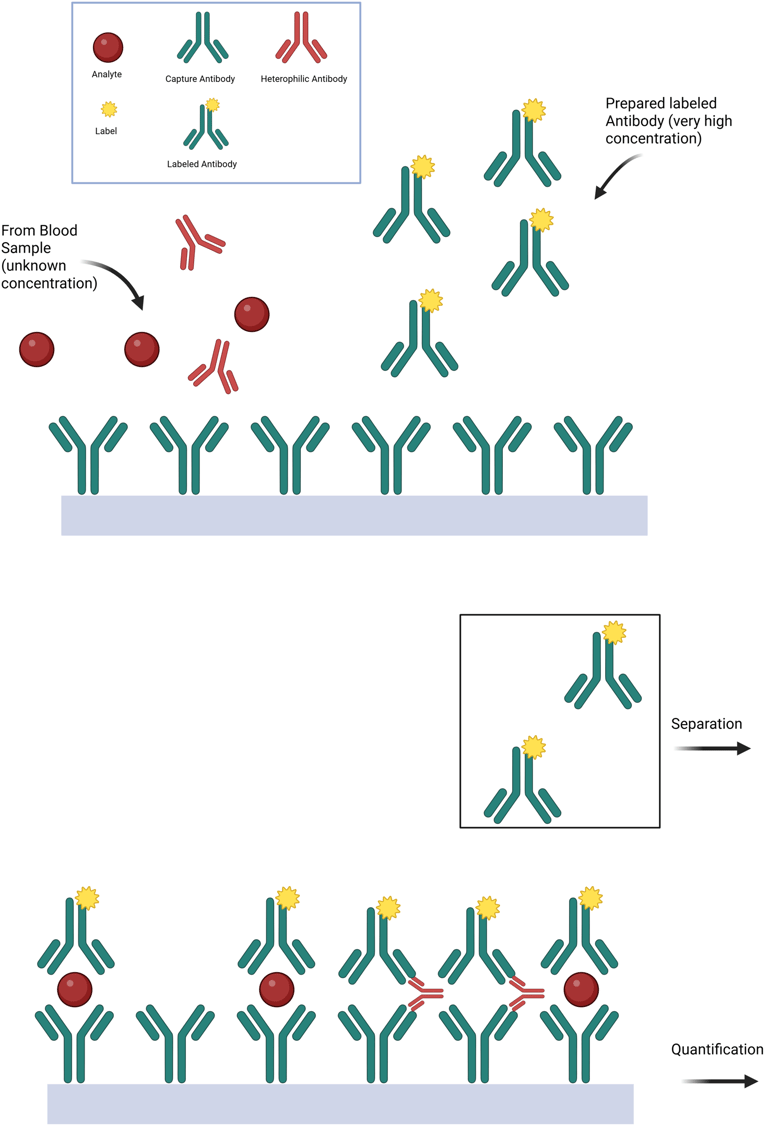
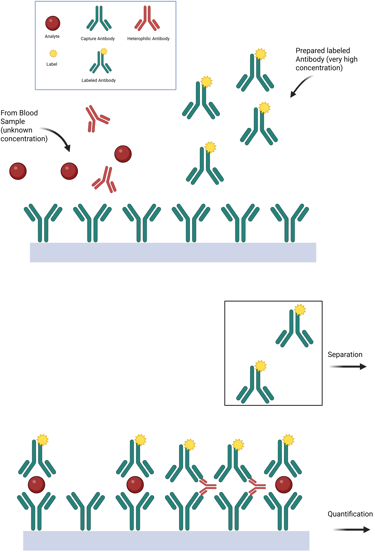
Illustration: Heterophile Antibody Interference
Non-Heterophile Antibody Interference with Falsely Low Results
Endogenous antibodies directed against the analyte itself can interfere with immunoassays by blocking the detection antibodies, thereby preventing accurate quantification and leading to falsely low or undetectable results. Unlike heterophile antibodies, these are analyte-specific and directly interfere with the assay’s ability to measure the target substance.
Non-Heterophile Antibody Interference with Falsely Elevated Results
Endogenous antibodies and binding proteins can also lead to falsely elevated test results. A notable example involves prolactin, which exists in multiple molecular forms: the biologically active monomeric prolactin (~23 kDa), along with dimeric prolactin (~50 kDa) and macroprolactin (>100 kDa), the latter being a complex of prolactin with immunoglobulin G (IgG). While macroprolactin is biologically inactive, it has preserved immunoreactivity and therefore is still detected by most immunometric assays, resulting in falsely elevated prolactin levels.
To detect macroprolactin interference, polyethylene glycol (PEG) precipitation is a widely used and cost-effective method. PEG removes macroprolactin by precipitation, allowing only monomeric prolactin to remain in the supernatant. A PEG-precipitable prolactin fraction exceeding 60% suggests significant macroprolactinemia, helping distinguish it from true hyperprolactinemia.
Macro-TSH represents another example of this phenomenon, potentially causing falsely elevated TSH levels.
Molecular Structure–Based Methods
Molecular Structure–Based Methods rely on the direct analysis of the chemical structure of analytes, providing a highly specific approach to hormone measurement. These techniques typically involve several steps, and various methods—such as extraction methods, chromatography, and mass spectrometry—can be used individually, in sequence or in combination with immunoassays. Unlike antibody-based methods, which estimate hormone concentrations through immunoreactivity, structure-based methods directly identify and quantify analytes based on their molecular mass and fragmentation patterns. This makes them especially valuable for measuring small molecules, such as steroid and thyroid hormones, where structural similarities and low concentrations may limit the accuracy of immunoassays.
Extraction methods involve transferring a substance from one phase to another based on differences in solubility. They are primarily used in sample preparation prior to quantification by mass spectrometry or, less commonly, immunoassays. Numerous extraction techniques exist, though their technical specifics are beyond the scope of this overview.
Historically, early steroid immunoassays often relied on extraction steps to reduce interference and improve specificity. These methods laid the foundation for understanding assay limitations. However, extraction prior to immunoassay is now rarely used in routine clinical practice, since these methods are difficult to automate,demand specialized expertise and equipment that are not widely available in clinical laboratories, and often necessitate recovery-based corrections to ensure accurate results.
In contrast, extraction remains a critical preanalytical step for mass spectrometry–based assays, where it enhances specificity and sensitivity. Significant advancements have been made in optimizing these extraction procedures for clinical application.
Chromatographic Systems use chromatographic separation of distinct biochemical forms, followed by the measurement of specific molecular properties for hormone quantification. Fundamentally, chromatography achieves analyte separation based on differential interactions with two phases: a stationary phase (typically a solid or, in gas chromatography, a liquid coated on a solid support) and a mobile phase (a liquid or gas) that carries the analytes through the stationary phase. As analytes pass through the stationary phase, their retention time—determined by their molecular structure, polarity, and solubility—serves as a distinguishing feature for identification. Quantification is typically achieved by integrating the peak area in the resulting chromatogram. Detection methods used in conjunction with chromatography vary and include UV/visible light absorption, fluorescence, and mass spectrometry (MS), the latter providing both structural identification and quantification.
Sample preparation techniques, as well as the composition of the mobile and stationary phases, are selected based on the properties of the target analyte to optimize separation and detection. Chromatography systems are often named based on the nature of the mobile and stationary phases. Commonly used systems are the following:
HPLC (High-Performance Liquid Chromatography): utilizes a liquid mobile phase under high pressure and is suitable for a wide range of analytes.
GC (Gas Chromatography): employs a gaseous mobile phase.
TLC (Thin Layer Chromatography): features a thin layer of stationary phase (e.g., silica gel) coated on a flat surface
These techniques offer two significant advantages:
Multiplex capability – They can simultaneously detect and quantify multiple forms of analytes.
Immuno-independence – They do not rely on immunologic reagents, reducing variability and enhancing harmonization across laboratories and platforms.
Limitations include their technical complexity, longer processing times, and limited accessibility in routine clinical laboratories due to the need for specialized equipment and expertise.
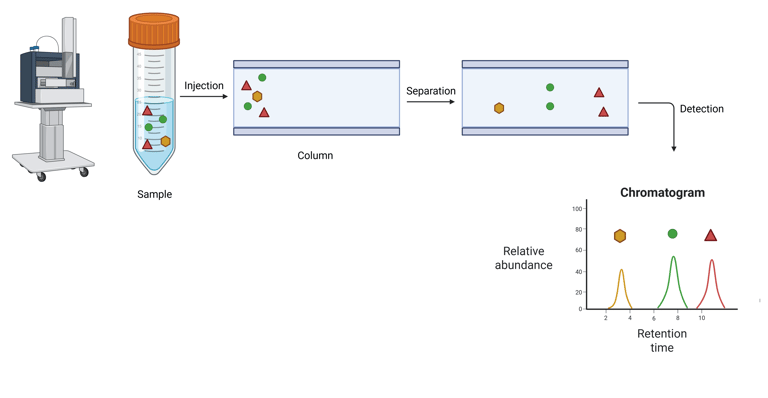
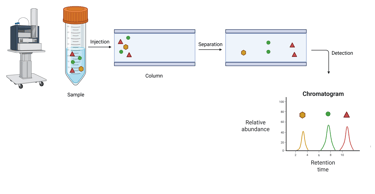
Illustration: Liquid Chromatography
Mass spectrometry is an analytical technique that enables the separation and quantification of molecules based on their mass-to-charge ratio (m/z). The fundamental principle involves the generation of charged particles (ions) from analytes, which are then directed through an electric or magnetic field. The resulting trajectory and behavior of these ions are governed by their specific m/z values, allowing for their separation and subsequent detection.
A mass spectrometer comprises a series of integrated components designed to facilitate this process. The instrument ionizes analytes, accelerates them into a mass analyzer that differentiates ions according to their m/z, and measures their relative abundance through a detector system.
Inlet System: Introduces the sample into the instrument. This can range from a straightforward injection port to more advanced systems, such as laser desorption or photo-ionization setups, depending on the analyte and sample type.
Ion Source: Converts the neutral analyte molecules into gas-phase ions, and in some cases, also induces fragmentation. This ionization is essential for subsequent mass analysis.
Mass Analyzer: The core analytical unit of the instrument. It uses electric and/or magnetic fields under vacuum conditions to separate ions according to their mass-to-charge ratios. Common analyzer types include quadrupoles, time-of-flight (TOF), and ion traps.
Detector (e.g., Faraday Plate or Electron Multiplier): Quantifies the separated ions by detecting the electrical current generated as ions collide with the detector surface. The magnitude of this signal is directly proportional to the ion abundance.
This highly specific and sensitive approach allows for the precise identification and quantification of analytes, making mass spectrometry a cornerstone in advanced bioanalytical and clinical diagnostics.
Clinical Relevance: The application of mass spectrometry in endocrine diagnostics has evolved significantly from its origins in analytical research. Initially, mass spectrometry required extensive purification and volatilization of analytes prior to ionization—a process particularly challenging for heat-labile compounds such as steroid hormones. These molecules often necessitated derivatization to enhance their thermal stability and ionization efficiency. While this methodology was initially confined to research settings, it played a pivotal role in elucidating the biochemistry of reproductive steroids.
The integration of gas chromatography (GC) with electron impact ionization mass spectrometry (GC/MS) marked a key transition into clinical application, particularly for the quantification of steroid hormones and other small endogenous molecules. However, as immunoassays emerged—offering lower cost and higher throughput—they rapidly supplanted GC/MS for routine endocrine testing in most clinical settings, except in large academic or reference laboratories.
More recently, technological advances in mass spectrometry, especially in ion source and mass analyzer designs, have led to the development of robust, automated instruments. These modern systems are increasingly being adopted in clinical laboratories, providing superior specificity, sensitivity, and multiplexing capabilities. As a result, mass spectrometry, particularly liquid chromatography-tandem mass spectrometry (LC-MS/MS), is now gaining prominence for hormone measurement, especially in scenarios where traditional immunoassays are limited by cross-reactivity or interference.
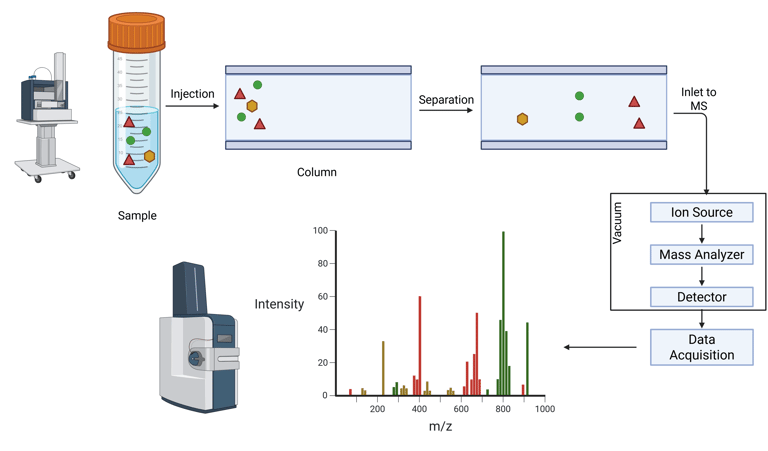
Illustration: Combined Liquid Chromatography and Mass Spectrometry
Methods for the Measurement of Free Hormones
As noted earlier, certain hormones, particularly steroid hormones, have a highly hydrophobic biochemical structure and circulate predominantly bound to plasma proteins such as albumin or specific transport proteins. According to the free hormone hypothesis, it is the unbound (free) fraction that exerts biological activity, as only free hormones are capable of diffusing into target tissues to initiate physiological effects. Under normal conditions, total and free hormone concentrations are proportionally related; however, this relationship may be altered when binding protein levels are abnormal.
Therefore, measuring free hormone levels can be clinically important, especially in conditions affecting binding protein concentrations. This has led to the development of specialized assays for assessing the free hormone fraction. These assays generally follow two approaches:
Physical separation of free and bound hormone prior to measurement: Dialysis
Antibody-based assays specifically designed to detect only the free hormone
Dialysis Method
Traditionally, equilibrium dialysis has been used to measure free hormone levels by separating free from protein-bound hormone using a semi-permeable membrane. This membrane, with pores sized to exclude larger molecules like binding proteins and hormone-protein complexes, allows only the free hormone to diffuse between two chambers—typically, the patient sample is placed on one side and a buffer solution on the other. Over time, the free hormone equilibrates across the membrane, enabling direct measurement in the dialysate.
Modifications of this method include ultrafiltration, which uses pressure to speed up separation without requiring equilibrium, and chemical precipitation, such as using ammonium sulfate to selectively precipitate binding proteins like SHBG. A major challenge in all these techniques is the very low concentration of free hormone in the separated fraction, making sensitive detection essential. To address this, some protocols incorporate a labeled hormone tracer prior to dialysis, enhancing the precision of quantification.
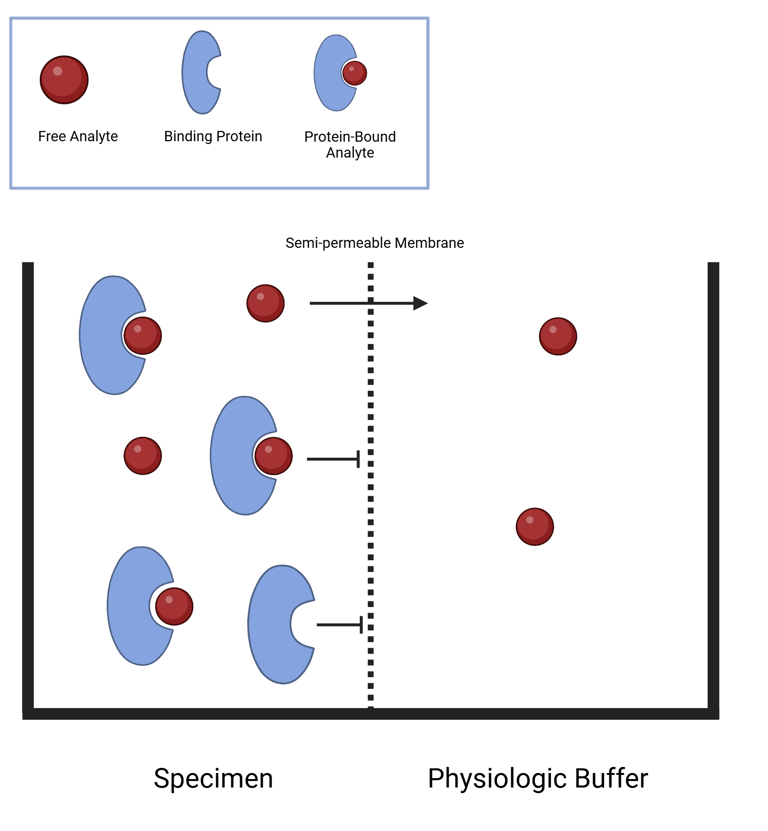
Illustration: Equilibrium Dialysis
Antibody-based binding assaysfor the measurement of free hormones can be divided into the following two classes: (1) two-step assays and (2) one-step assays.
In two-step immunoassays, free hormone levels are estimated using a solid-phase antibody that captures the unbound fraction of hormone from the specimen. The method relies on the relative binding affinities of the antibody and endogenous binding proteins. If the antibody has higher affinity, it may displace the hormone from its binding protein; if lower, it will bind only the free fraction. After the specimen is incubated and washed, labeled hormone is added to titrate the remaining unoccupied antibody sites. After a second washing step, the signal from this labeled hormone is measured and allows for analyte quantification, as it is inversely proportional to the amount of free hormone originally present. Importantly, the definition of “free” in this assay depends on the antibody's relative affinity compared to the hormone's natural binding proteins.
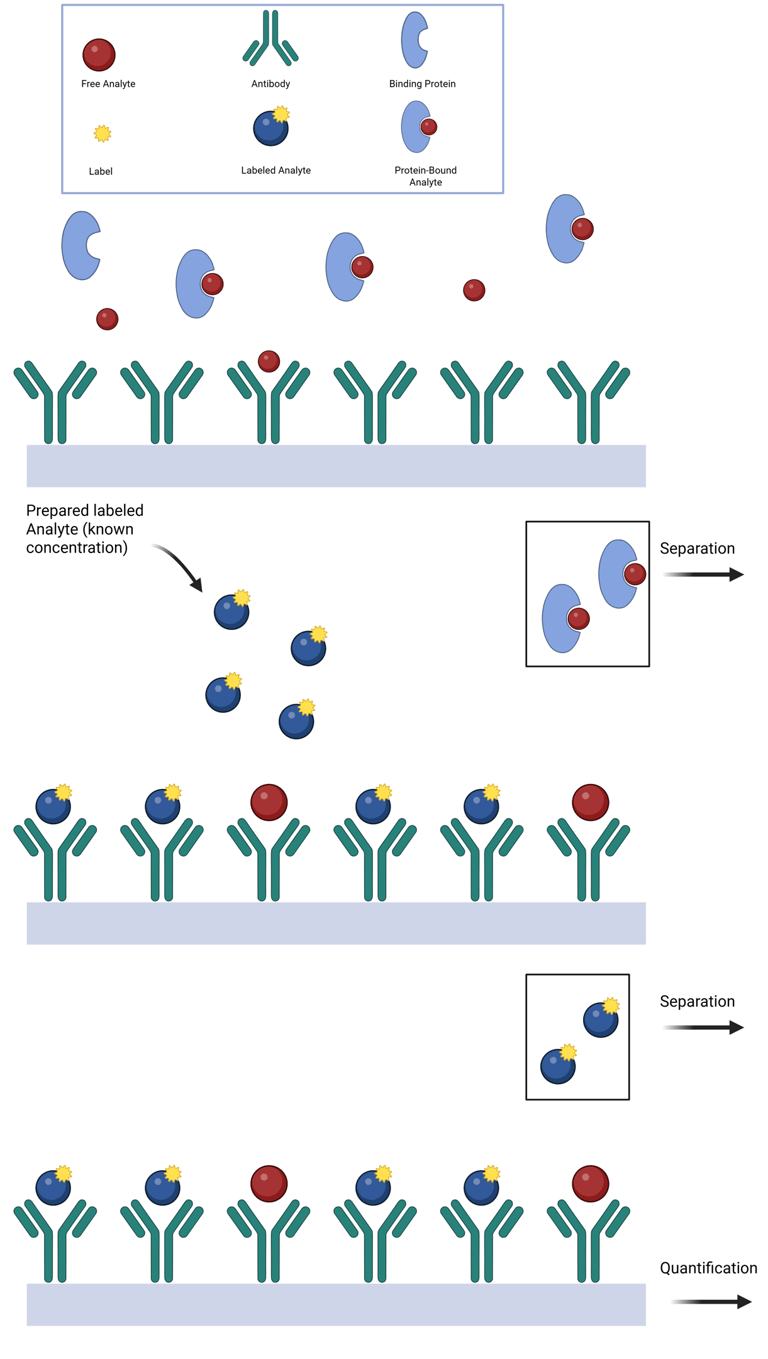
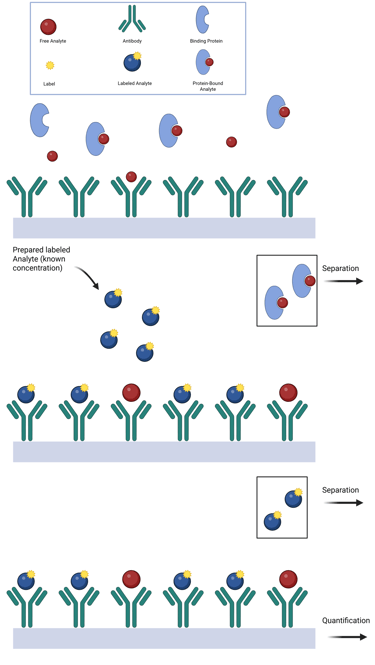
Illustration: Two Step Immunoassay
One-step immunoassays utilize either a labeled steroid analogue or a labeled antibody and are designed for simplicity, speed, and ease of automation. In the labeled steroid format, the analogue competes with free hormone in the specimen for antibody-binding sites on a solid phase. Because the analogue is not recognized by binding proteins, the assay assumes that the signal, inversely proportional to free hormone concentration, reflects only this competition. However, this assumption may not hold universally; for example, it has proven unreliable for free testosterone assays and only conditionally valid for free thyroxine, depending on binding protein levels.
An alternative format uses a labeled antibody that binds to a solid-phase antigen (e.g., the hormone of interest). At equilibrium, the signal generated reflects the inverse of the free hormone concentration in the specimen. This approach offers improved sensitivity and precision, without requiring chemical modification of the hormone (except for immobilization to the solid phase).
Summary: Criteria for a Good Laboratory Test in Hormone measurement
1. Biochemical/Physiological Requirements
Measurable & stable hormone: The hormone must be stable in the selected specimen type, and the assay must be sensitive enough to detect concentrations within both physiological and pathological ranges.
Known/reproducible secretion patterns: The hormone should have a well-characterized secretion profile to allow meaningful interpretation and detection of regulatory disturbances (e.g., circadian rhythm, pulsatility).
2. Preanalytical Phase
Correct patient identification: Ensure accurate patient ID to avoid sample mislabeling or mix-ups.
Appropriate test ordering: Select the test most suited to answer the clinical question or diagnostic suspicion.
Timing & preparation: Account for diurnal variations, fasting status, recent physical activity, and medication use that may affect hormone levels.
Correct specimen type: Choose the appropriate matrix—serum, plasma, urine, saliva—based on the hormone’s characteristics and assay validation.
Proper sample collection: Use the correct tubes and additives, follow proper phlebotomy technique and order of draw.
Sample handling & transport: Ensure proper temperature control, minimize preanalytical delays, and protect specimens from degradation or contamination.
3. Analytical Phase
Test availability & validation: The assay should be validated for clinical use, with established accuracy, precision, and reliability.
Standardized methods: Employ harmonized protocols and calibrated assays to enable comparability across laboratories.
Reference intervals & cutoffs: Use population-specific and specimen-specific reference ranges, verified for the particular method and matrix.
4. Postanalytical Phase
Accurate report generation: Clearly report values with appropriate units, reference ranges, and any relevant interpretive comments.
Interpretation in clinical context: Always interpret results considering the clinical picture, medication use, and biological variability (e.g., pulsatile secretion, half-life, binding protein levels).
Communication: Provide meaningful, actionable conclusions, highlight critical results, and suggest follow-up or repeat testing if indicated.
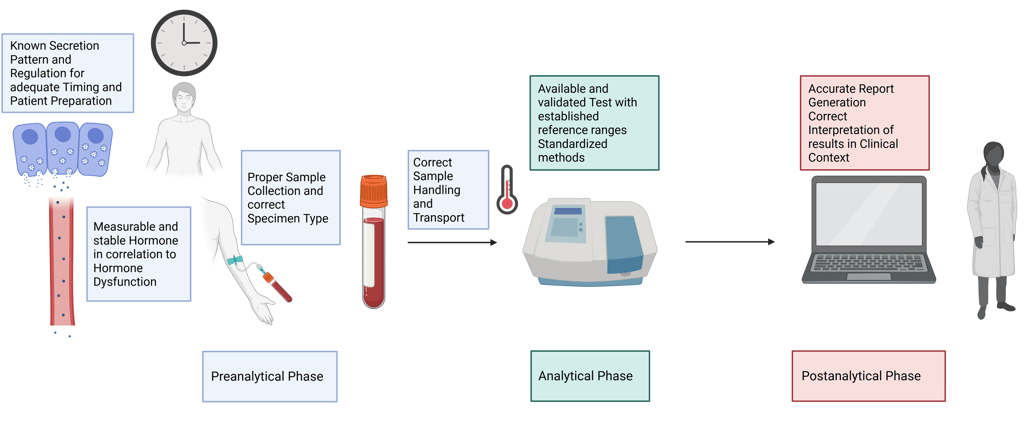
Illustration Criteria for a Good Laboratory Test in Hormone measurement
References
All Illustrations were created in https://BioRender.com
For References, visit the Section "References" in General Principles of Clinical Endocrinology
© 2025 EndoCases. All rights reserved.
This platform is intended for medical professionals, particularly endocrinology residents, and is provided for educational purposes only. It supports learning and clinical reasoning but is not a substitute for professional medical advice or patient care. The information is general in nature and should be applied with appropriate clinical judgment and in accordance with local guidelines.
Portions of the text on this website were edited with the assistance of Artificial Intelligence to improve grammar and phrasing, as English is not my first language. All medical content, ideas for illustrations, and overall structure are original and based on the author’s own expertise and the cited medical literature. No AI tools were used to generate or influence the educational content itself.
All of the content is independent of my employer.
Use of this site implies acceptance of our Terms of Use
Contact us via E-Mail: contact@endo-cases.com