Clinical Implications of Physiological and Pathological Hormone Synthesis and Secretion
Hormones are synthesized by a diverse array of specialized cells distributed throughout multiple organs in the human body. While some hormones are synthesized using standard protein production mechanisms common to most cells, others require unique enzymes and specialized synthetic pathways found only in certain endocrine tissues. The production of certain hormones rely on precursor molecules—such as cholesterol for steroid hormones or tyrosine for catecholamines—or additional substrates, like iodide in the production of thyroid hormones.
The functional development of endocrine cells begins in utero and involves intricate spatiotemporal regulation of pluripotent stem cells through lineage-specific transcription factors and locally acting factors. As differentiation progresses, endocrine cells in various glands typically become specialized to produce and secrete a specific hormone (see Table 1-1). There are exceptions, such as the mammosomatotroph cells of the anterior pituitary, which can co-secrete or alternate between growth hormone and prolactin secretion. Endocrine cells are thus classified according to both their developmental lineage and the profile of hormones they produce.
Clinical Implications: The ultimate function of endocrine cells and glands is determined by genetically regulated developmental processes that govern the synthesis of the cellular machinery required for hormone production and secretion. Mutations in genes involved in these pathways can disrupt hormone biosynthesis, secretion, or action, leading to a wide spectrum of endocrine dysfunctions. Recognizing these genetic foundations is essential for accurate diagnosis, selecting appropriate therapeutic strategies and for providing effective patient counseling regarding prognosis, treatment expectations, and potential hereditary implications. A classic example of the clinical relevance of cellular signaling mechanisms is monogenic diabetes mellitus. Frequently misclassified as other forms of diabetes, this condition is caused by mutations in genes that regulate glucose sensing or the intracellular pathways governing insulin synthesis and secretion. Accurate diagnosis is essential, as it significantly influences both the prognosis and the choice of appropriate treatment, which can differ markedly from standard diabetes therapies.
Furthermore, unique cellular mechanisms involved in hormone synthesis offer important clinical opportunities. For instance, the processes governing iodide uptake and metabolism—crucial for thyroid hormone production—form the basis for using technetium in imaging and radioactive iodine in both diagnosis and therapy.
Additionally, specific endocrine cell lineages exhibit varying degrees of vulnerability to particular pathological insults. Certain noxious stimuli—whether genetic, autoimmune, inflammatory, ischemic, or toxic—may preferentially affect onecell type within a gland, leading to selective functional impairments. For example, in type 1 diabetes mellitus, autoimmune destruction predominantly targets insulin-producing β-cells in the pancreatic islets, while other islet cell types remain relatively unaffected.
Table 1.1 Endocrine Cell Types and Their Corresponding Hormones
Endocrine secretory cells typically contain intracellular granules that store substantial quantities of hormone, allowing for rapid and controlled release in response to specific physiological signals. While the secretion of a hormone does not solely determine its ultimate biological effect—since this depends on receptor availability and downstream signaling pathways—it is nevertheless a fundamental prerequisite for endocrine function. Because the efficacy of an endocrine system depends on both the adequate synthesis and timely release of its hormones, these processes are subject to precise regulatory control. In general, hormonal secretion is governed by an intricate interplay of electrochemical and biochemical signals, ensuring appropriate responsiveness to internal and external stimuli.
While the regulation of certain endocrine systems can be relatively straightforward, for instance, parathyroid hormone (PTH) synthesis is directly modulated by circulating ionized calcium, the very substance it regulates, other systems exhibit considerably more complex, multi-tiered control mechanisms. A prominent example is the hypothalamic-pituitary-peripheral axis, in which hierarchical regulatory pathways coordinate hormone release to exert specific effects on distant target organs. Although we will examine the various axes under hypothalamic and pituitary regulation in greater detail later, we introduce the regulatory architecture here by using the hypothalamic-pituitary-adrenal (HPA) axis to illustrate the system’s inherent complexity.
Illustration of Ca Regulation through PTH. Parathyroid hormone (PTH) is secreted by the chief cells of the parathyroid glands. Its primary regulatory mechanism is the concentration of ionized calcium in the blood, which exerts a negative feedback effect by suppressing PTH secretion. Additional regulatory influences include activated Vitamin D and Fibroblast Growth Factor 23 (FGF-23), both of which inhibit PTH secretion, as well as serum phosphate, which acts as a stimulatory factor. Once released, PTH circulates to various tissues. Although its receptors are expressed widely throughout the body, its principal target organs for calcium homeostasis are the bones and kidneys. PTH promotes bone resorption, enhances renal calcium reabsorption, and stimulates the activation of vitamin D. Activated vitamin D, in turn, increases intestinal absorption of calcium. Collectively, these direct and indirect actions of PTH result in elevated serum calcium levels, which subsequently inhibit further PTH release through negative feedback at the level of the parathyroid glands. The kidney and liver are the primary organs responsible for the metabolism and clearance of parathyroid hormone, with additional contributions from bone and muscle tissues.
Created in https://BioRender.com
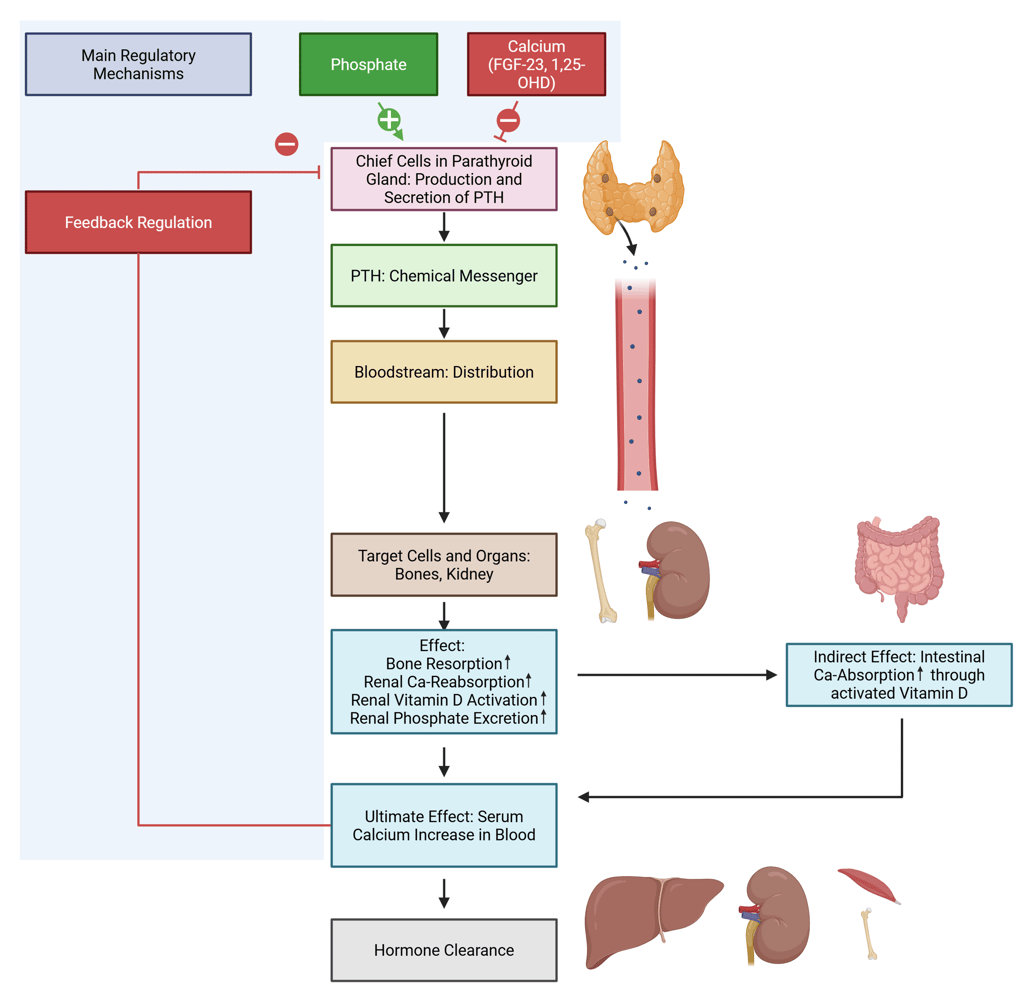
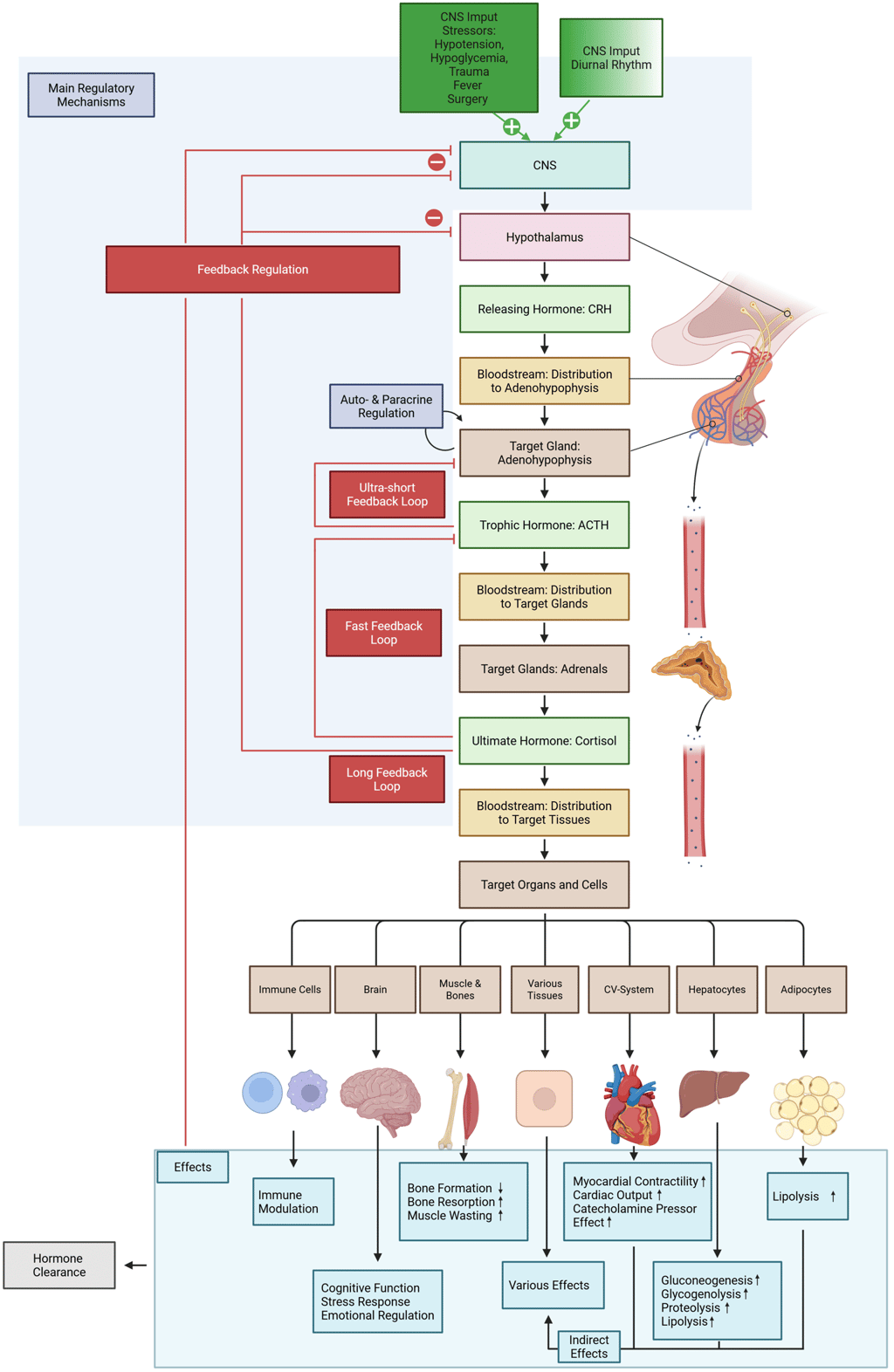
Illustration of the HPA-axis: The hypothalamus functions as the central integrator of endocrine regulation within the hypothalamic-pituitary-adrenal (HPA) axis by secreting corticotropin-releasing hormone (CRH), a tropic hormone that regulates the activity of anterior pituitary corticotrophs. Hypothalamic CRH secretion is modulated by both internal physiological states and external environmental cues. These influences are transmitted via electrochemical signals from the central nervous system—such as those associated with stress or sensory input (e.g., circadian rhythms synchronized by light exposure)—as well as through biochemical feedback mechanisms. Predominant among these are circulating hormones, particularly cortisol, which exerts negative feedback on CRH secretion.
CRH enters the hypothalamic-pituitary portal circulation and binds to specific transmembrane receptors on anterior pituitary corticotrophs, stimulating the release of adrenocorticotropic hormone (ACTH). ACTH secretion is further regulated by paracrine factors such as cytokines and growth factors and may also be modulated via ultra-short autocrine feedback loops. Finally, ACTH stimulates the secretion of cortisol in the adrenal cortex through binding to its specific receptor.
Cortisol is ultimately the final effector hormone of the HPA axis. It exerts its effects both directly—by acting on distant target tissues—and indirectly, primarily through metabolic modulation. As previously noted, it also participates in negative feedback loops that inhibit both hypothalamic CRH and pituitary ACTH secretion, thereby maintaining homeostasis. Some downstream effects of cortisol contribute to feedback regulation as well. For instance, the cortisol-mediated resolution of hypoglycemia—via enhanced gluconeogenesis and glycogenolysis—helps suppress further activation of the HPA axis. This multilayered control system enables precise neuroendocrine integration of internal and external stimuli, ensuring balanced hormonal responses and preventing both hypo- and hypercortisolism. Cortisol clearance primarily involves the liver and kidney as the main organs, with key enzymatic activity (mainly 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2), which inactivates cortisol to cortisone) in specific cell types within these tissues.
Created in https://BioRender.com
Additionally, certain endocrine systems exhibit specialized regulatory mechanisms in response to specific physiological conditions. For instance, the thyroid gland downregulates hormone synthesis upon exposure to excessive iodine through a process known as the Wolff–Chaikoff effect, which serves to prevent iodine-induced hormone hypersecretion.
As a result of the complex regulatory mechanisms governing endocrine function, many hormones are secreted in distinct temporal patterns that optimize their physiological effects on target tissues. These patterns—ranging from circadian to pulsatile and episodic—reflect the integration of neuroendocrine, metabolic, and environmental inputs, and are crucial for maintaining homeostasis.
Circadian secretion is characteristic of several pituitary hormones, most notably adrenocorticotropic hormone (ACTH) and its downstream effector cortisol, both of which exhibit a pronounced peak in the early morning hours, typically before 9:00 AM. Similarly, approximately 70% of growth hormone (GH) release occurs during slow-wave sleep, and age-related reductions in sleep quality contribute to decreased GH and elevated nocturnal cortisol levels.
Combined rhythmic and state-dependent secretion is observed in hypothalamic releasing hormones such as growth hormone-releasing hormone (GHRH), which follows a circadian rhythm in the amplitude and frequency of its pulsatile bursts, while also responding to metabolic signals such as fasting or hypoglycemia.
Pulsatile secretion is a hallmark of several hormones, including insulin, which is released in biphasic pulses. The first phase consists of a rapid release of preformed hormone, followed by a more sustained second phase driven by continued nutrient stimulation and other modulatory signals.
Continuous secretion is more characteristic of certain hormones such as thyroxine (T4) and triiodothyronine (T3), which are released steadily to maintain relatively stable circulating levels due to their long half-lives and broad systemic effects.
Episodic secretion, exemplified by gonadotropins (LH and FSH), is governed by the hypothalamic pulse generator and feedback regulatory systems. It is critical for coordinating reproductive function, particularly the menstrual cycle in females.
Clinical Implications of Endocrine Regulation Mechanisms
An understanding of the physiological regulatory mechanisms governing hormone secretion is essential for accurate clinical interpretation and decision-making in endocrinology. To begin with, adequate hormone secretion is a critical determinant of sufficient hormone action; disruption of this process leads to functional imbalances, including overproduction or underproduction, with resulting clinical and biochemical consequences.
The hierarchical organization of endocrine axes not only allows detection of hormonal dysfunction but also helps localize its origin. For example in regulatory feedback loops between the hypothalamic-pituitary axis and a peripheral endocrine gland, the concentration of the pituitary-derived tropic hormone—such as TSH for the thyroid or ACTH for the adrenal cortex—serves as a highly sensitive marker of underactive or overactive target gland function. Characteristic hormone patterns enable differentiation between primary dysfunction (originating in the target gland) and secondary or tertiary (central) causes at the pituitary or hypothalamic level. For example, low cortisol accompanied by elevated ACTH is indicative of primary adrenal insufficiency, whereas low cortisol with low ACTH suggests secondary or tertiary (central) insufficiency. This layered structure also underpins the rationale for dynamic function testing. Stimulation tests such as the insulin-induced hypoglycemia test assess the integrity of the hypothalamic-pituitary-adrenal (HPA) axis by provoking CRH and ACTH secretion, while the cosyntropin stimulation test evaluates adrenal responsiveness directly.
Additionally, hormone concentrations that fall outside standard reference ranges do not necessarily reflect pathological states. Instead, they may represent appropriate compensatory responses to physiological stressors or metabolic imbalances. For example, elevated PTH in secondary hyperparathyroidism often reflects an adaptive response to chronic hypocalcemia or vitamin D deficiency, rather than primary parathyroid pathology. Similarly, chronic illness or stress can reset regulatory setpoints, as observed in non-thyroidal illness syndrome, leading to transient hormonal abnormalities that should not be misinterpreted as primary endocrine disease.
Moreover, hormone levels may fall within the reference range yet still be inappropriate in the physiological context. For example, in hypocortisolism, a markedly elevated ACTH concentration would typically be expected as a compensatory response. The absence of such an elevation suggests an impaired regulatory mechanism, pointing to secondary or tertiary adrenal insufficiency, as discussed above. Finally, since the secretion of many hormones follows circadian, pulsatile, or situational patterns, shaped by the summation of regulatory inputs, accurate hormone measurement, requires consideration of timing and physiological context. For instance, basal cortisol levels are most diagnostic for hypocortisolism when measured in the early morning, reflecting their diurnal peak. Whereas insulin levels are most informative, in regard to secretion adequacy, when assessed during hypoglycemic episodes. Random or untimed measurements can be misleading in the case of hormones with significant temporal variation, such as ACTH, growth hormone, and LH/FSH, making dynamic or serial sampling essential in some diagnostic settings. In some cases, hormone overproduction can disrupt normal diurnal rhythms, as seen in conditions like Cushing's syndrome. This necessitates diagnostic approaches that capture integrated hormone secretion over time—such as late-night salivary cortisol or 24-hour urinary free cortisol measurements—rather than relying on single time-point sampling.
Together, these principles highlight the importance of integrating physiological knowledge into clinical endocrine assessment. They not only inform the interpretation of hormone levels but also guide test selection, timing, and the differentiation between adaptive, functional, and pathological processes. This neuroendocrine framework ensures more precise diagnostics and avoids misdiagnosis or overtreatment based on decontextualized laboratory data.
References
All Illustrations were created in https://BioRender.com
For References, visit the Section "References" in General Principles of Clinical Endocrinology
© 2025 EndoCases. All rights reserved.
This platform is intended for medical professionals, particularly endocrinology residents, and is provided for educational purposes only. It supports learning and clinical reasoning but is not a substitute for professional medical advice or patient care. The information is general in nature and should be applied with appropriate clinical judgment and in accordance with local guidelines.
Portions of the text on this website were edited with the assistance of Artificial Intelligence to improve grammar and phrasing, as English is not my first language. All medical content, ideas for illustrations, and overall structure are original and based on the author’s own expertise and the cited medical literature. No AI tools were used to generate or influence the educational content itself.
All of the content is independent of my employer.
Use of this site implies acceptance of our Terms of Use
Contact us via E-Mail: contact@endo-cases.com