Clinical Implications of physiological and pathological Hormone Signaling
Hormones can be broadly classified into two categories—tropic and non-tropic—based on their primary mode of action. Tropic hormones primarily target other endocrine glands, stimulating them to synthesize and secrete additional hormones and often as well have an effect on growth and proliferation of target gland cells. In contrast, non-tropic hormones exert their effects directly on specific tissues or cells without involving intermediary endocrine structures.
Tropic hormones play a pivotal role in regulating the activity of downstream endocrine glands. Notable examples include thyroid-stimulating hormone (TSH), the gonadotropins—luteinizing hormone (LH) and follicle-stimulating hormone (FSH) and the earlier mentioned adrenocorticotropic hormone (ACTH). By promoting the release of secondary hormones from their target glands, tropic hormones are key regulators of essential physiological processes such as metabolic rate, stress adaptation, and reproductive function.
In contrast, non-tropic hormones act directly upon their target tissues without mediating effects through other endocrine glands. Examples include insulin and prolactin.
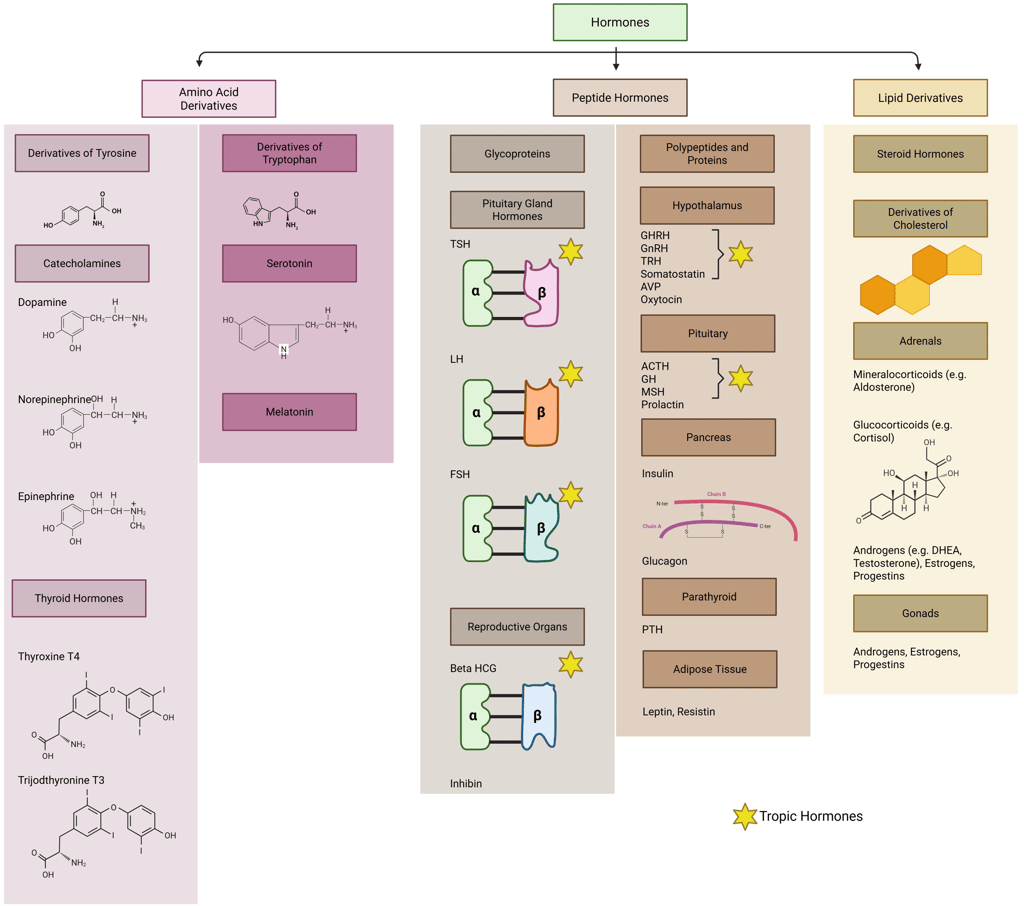
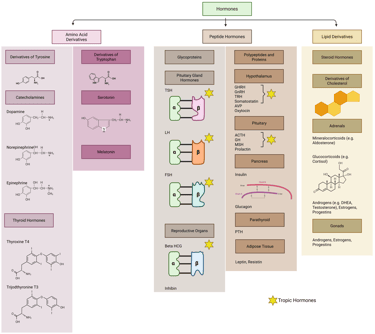
Illustration: Tropic and Non-Tropic Hormones
Signal Transduction
The primary role of hormones is to transmit information from the extracellular environment into the cell’s interior to regulate cellular activities. This process is referred to as signal transduction. At its core, the mechanism can be divided into four key stages:
Hormone Binding
Intracellular Signaling
Resulting Effect
Termination of the Signal
Based on their mechanism of action, hormones initiate signal transduction through two principal pathways:
Intracellular Receptor Binding: Certain hormones cross the plasma membrane, either by passive diffusion or active transport, and bind to intracellular receptors—typically nuclear receptors—to directly regulate gene transcription. Classic examples include thyroid hormones and steroid hormones.
Cell Surface Receptor Binding: Other hormones exert their effects by binding to specific receptors on the cell surface, without crossing the cell membrane. In these cases, signal transduction can range from relatively simple mechanisms, such as altering the cell’s electrochemical environment via ligand-gated ion channels, to more complex cascades involving multiple intracellular steps that transmit the signal to downstream effectors (see below). Hormones in this category include polypeptides, glycoproteins, and monoamines (such as serotonin).
Hormone Binding: The process begins with the hormone (ligand) binding to its specific receptor. This receptor can be located on the cell surface (e.g., G-protein-coupled receptors) or within the cell (e.g., nuclear receptors) (see below).
Receptor Activation: Upon binding, the receptor undergoes a conformational change that activates it. For cell surface receptors, this often involves the activation of associated G-proteins or receptor tyrosine kinases.
Signal Transduction: The activated receptor initiates a cascade of intracellular signaling pathways to synthesize second messengers.
Second Messenger Signaling: Second messengers amplify the signal within the cell. For example, cAMP activates protein kinase A (PKA), while IP3 triggers the release of calcium from intracellular stores.
Activation of Downstream Effectors: Second messengers activate various downstream effectors, including kinases and phosphatases, which further propagate the signal by phosphorylating target proteins that regulate integral, metabolic and/or transcriptional functions within the cell.
Termination of Signal: The signal is terminated through various mechanisms, including receptor desensitization, internalization, degradation, and the deactivation of second messengers.
Ligand-Gated Ion Channels
Among the simplest types of cell surface signaling mechanisms are ligand-gated ion channels, in which a single protein or protein complex fulfills both the hormone-binding and signal-transducing roles. These channels consist of two main components: a ligand-binding domain accessible from the cell surface and a transmembrane domain forming an ion-conducting pore. When a ligand binds to the extracellular domain, it induces a conformational change to open the channel, allowing ions—such as calcium—to pass across the plasma membrane.
Although this mechanism illustrates the fundamentals of signal transduction, ligand-gated ion channels play only a limited role in clinical endocrinology, as most hormonally active pathways rely on more complex intracellular signaling cascades (see below). Generally, ligand-gated ion channels function primarily as neurotransmitter receptors, supporting the need for rapid, millisecond-scale responses at synapses. One exception in endocrine physiology involves receptors for certain hypothalamic releasing hormones. For instance, serotonin may modulate prolactin secretion by activating 5-HT3 receptors on lactotrophs in the anterior pituitary.
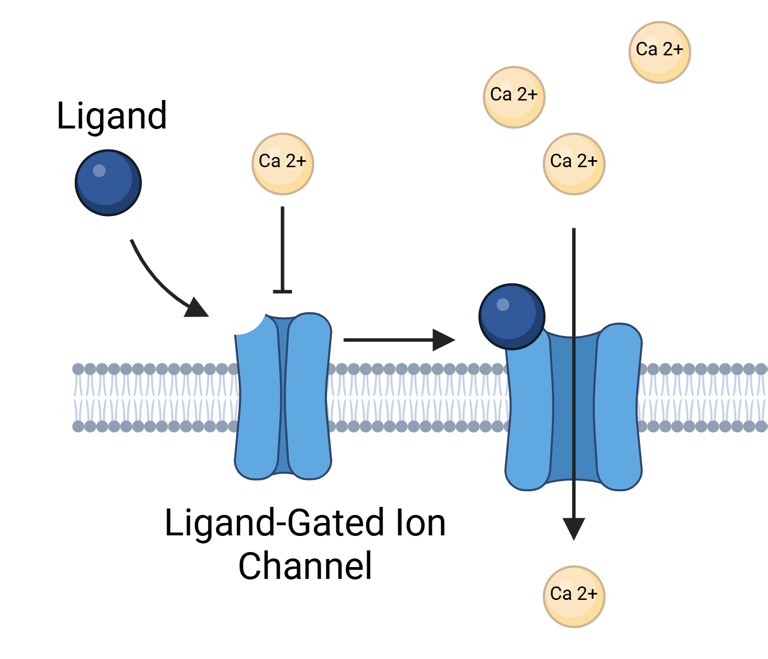
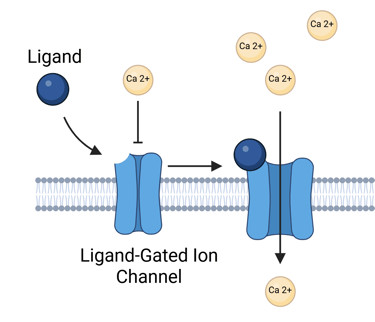
Illustration Ion Gated Ion Channels
G Protein–Coupled Receptors (GPCRs): Structure and Signaling Mechanism
G protein–coupled receptors (GPCRs) constitute the largest and most diverse class of cell surface receptors, characterized by their interaction with heterotrimeric G proteins. These receptors share a conserved structure comprising seven transmembrane α-helices—each approximately 25 amino acids in length—earning them the designation "seven-transmembrane (7TM)" proteins. Structurally, the N-terminal domain is extracellular, while the C-terminal tail resides within the cytoplasm.
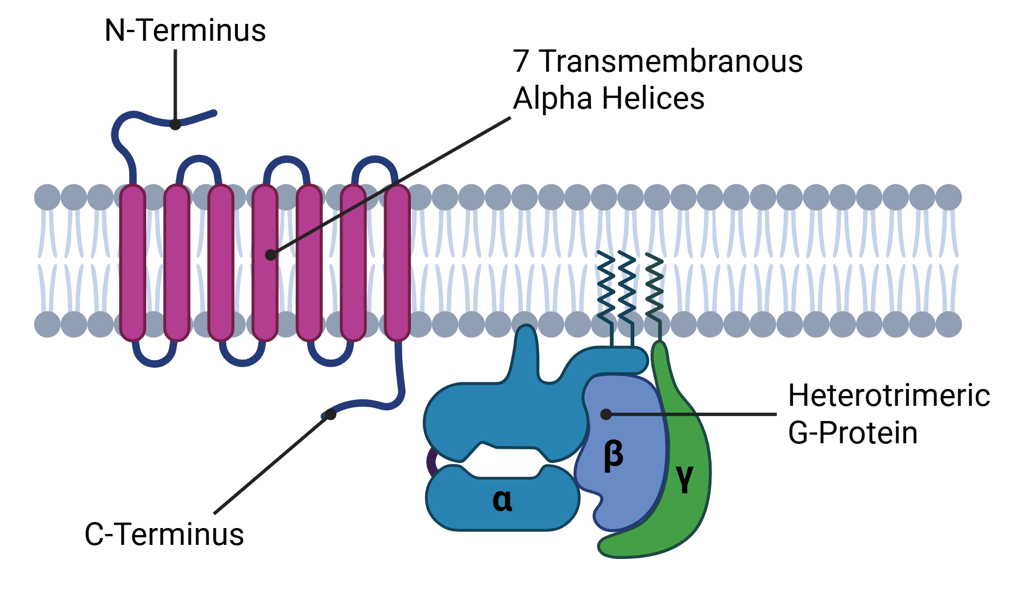
Illustration: Structure of G-Protein Coupled Receptors (GPCR)
Genome-wide analyses have identified over 800 GPCR genes in humans, the majority of which encode olfactory receptors. However, a broad array of physiologically active hormones, including catecholamines, glucagon, parathyroid hormone (PTH), calcitonin, and several hypothalamic releasing hormones, signal through GPCRs. Small ligands, such as catecholamines, typically bind within the transmembrane region, while larger peptide hormones interact with both the extracellular N-terminus and transmembrane domains.
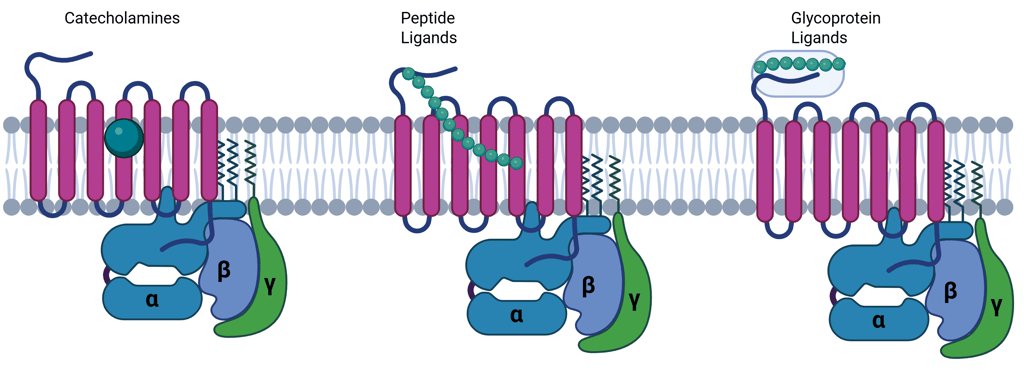
Mechanism of Signal Transduction via G Protein Coupled Receptors
After ligand binding, GPCRs undergo conformational changes that promote coupling with heterotrimeric G proteins composed of α, β, and γ subunits. These G proteins are preassembled with effector proteins on the inner surface of the plasma membrane. Ligand engagement facilitates the release of GDP from the Gα subunit, allowing GTP—present in higher intracellular concentrations—to bind. This GTP-loading activates the Gα subunit, which then dissociates from the βγ dimer, initiating downstream signaling events.
There are approximately 20 Gα subunit variants encoded by 16 genes, classified into four functional families:
Gs, which stimulates adenylyl cyclase;
Gi, which inhibits adenylyl cyclase;
Gq/11, which activates phospholipase C-β (PLCβ), generating second messengers inositol triphosphate (IP₃) and diacylglycerol (DAG);
G12/13, involved in cytoskeletal remodeling.
Illustration: GPCR Binding sites
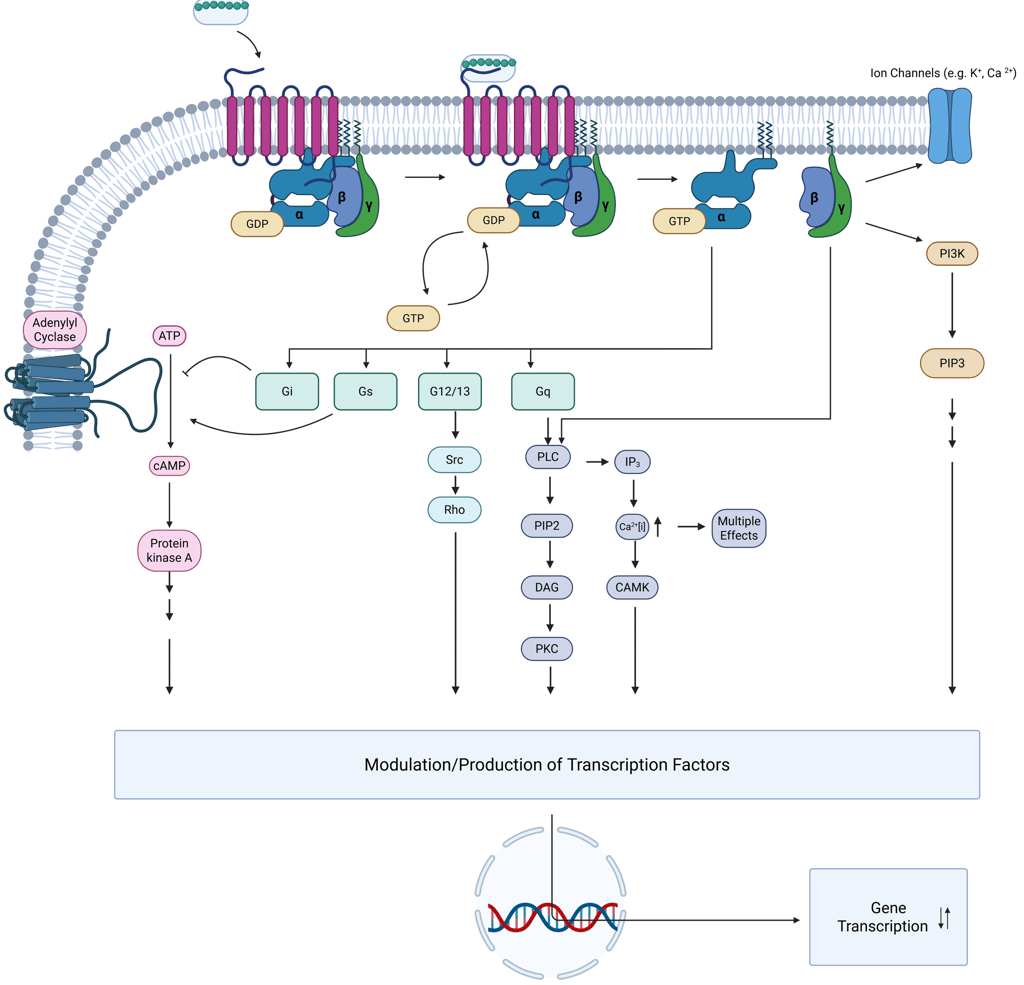
A key feature of G protein signaling is its transient nature, functioning as a "molecular switch." The signal remains active while GTP is bound to the Gα subunit. Termination occurs when the intrinsic GTPase activity of Gα hydrolyzes GTP to GDP, prompting reassembly of the heterotrimer and cessation of downstream signaling. This Process can be regulated by other proteins, such as GPCR scaffolding proteins and Arrestins.
After activation, GPCRs themselves are subject to regulatory processing. They may be internalized into endosomes and either recycled back to the plasma membrane or directed toward lysosomal degradation, thus modulating receptor availability and cell sensitivity to subsequent stimuli.
Clinical Relevance. While the detailed molecular intricacies of G protein–coupled receptor (GPCR) signaling are not essential for day-to-day clinical practice in endocrinology, a foundational understanding of the principles of hormone signal transduction remains highly relevant. GPCRs represent the largest class of membrane-bound hormone receptors, and the pathways they activate explain many context-dependent effects of hormone action. A single hormone can exert diverse physiological outcomes depending on the receptor subtype it binds to and the downstream signaling pathways activated within a specific target cell. For example, vasopressin acts via V1 receptors (coupled to Gq proteins) to induce vasoconstriction, while V2 receptors (coupled to Gs proteins) mediate renal water reabsorption—illustrating how signal transduction mechanisms, rather than the hormone itself, dictate cellular responses.
Clinically, several endocrine disorders are directly linked to defects in GPCRs or their associated signaling pathways. Inactivating mutations in the calcium-sensing receptor (CaSR), a GPCR, lead to familial hypocalciuric hypercalcemia, whereas activating mutations in the TSH receptor contribute to autonomously functioning thyroid nodules. Disorders like pseudohypoparathyroidism arise from resistance to hormone action due to mutations in downstream signaling molecules such as the Gsα subunit of the heterotrimeric G protein complex. Similarly, McCune-Albright syndrome is caused by somatic activating mutations in GNAS, leading to constitutive Gs signaling and endocrine hyperfunction.
Understanding GPCR signaling also has practical therapeutic implications. Many pharmacologic agents used in endocrinology target these receptors, including somatostatin analogs used in the treatment of acromegaly and neuroendocrine tumors, and dopamine agonists such as cabergoline for prolactinomas. Knowledge of receptor signaling mechanisms aids in predicting drug efficacy, potential resistance, and side effect profiles.
Thus, a conceptual grasp of signal transduction via GPCRs not only enhances understanding of endocrine pathophysiology but also supports more precise clinical reasoning, diagnosis, and management.
Illustration: Signal Transduction via GPCR
Signaling Through Tyrosine Protein Kinase
Hormonal signaling through tyrosine kinases involves two principal classes of receptors: those with intrinsic tyrosine kinase activity and those that rely on association with intracellular, non–membrane-spanning tyrosine kinases.
1. Receptor Tyrosine Kinases (RTKs)
Receptors in the RTK family are integral membrane proteins that possess intrinsic enzymatic activity. These receptors function by converting extracellular hormone binding into intracellular enzymatic activation. Structurally, RTKs are type I transmembrane proteins composed of an extracellular N-terminal ligand-binding domain, a single hydrophobic transmembrane segment of approximately 25 amino acids, and a cytoplasmic C-terminal region containing a tyrosine kinase domain. Upon ligand engagement—such as by insulin, insulin-like growth factor 1 (IGF-1), fibroblast growth factors (FGFs), or epidermal growth factor (EGF)—the receptor undergoes conformational change and dimerization, leading to phosphorylation (including autophosphorylation of the receptor) on specific tyrosine residues. This phosphorylation cascade initiates downstream signaling pathways by recruiting and activating intracellular signaling proteins, ultimately influencing processes such as cell metabolism, growth, and differentiation through both genomic and non-genomic effects. For a hormone like insulin to exert its physiological effects, several sequential steps are required: (1) hormone recognition by the receptor, (2) conformational modification of the receptor, (3) transduction of the extracellular signal across the plasma membrane, and (4) activation of intracellular signaling pathways.
2. Receptors Associated with Tyrosine Kinases
In contrast to RTKs, another class of receptors lacks intrinsic kinase activity but transduces signals via association with intracellular tyrosine kinases, such as Janus kinases (JAKs). These receptors, exemplified by the growth hormone receptor (GHR), erythropoietin receptor (EPO-R), prolactin receptor, and thrombopoietin receptor, depend on ligand-induced dimerization for activation. Typically, the hormone ligand contains two distinct binding motifs that engage different extracellular regions of the receptor dimer, bringing two associated JAK kinases into proximity. This spatial arrangement allows for reciprocal (trans-)phosphorylation of both the receptor and the associated JAKs, thereby initiating intracellular signaling. Key signaling mediators recruited to these phosphorylated receptors include phosphoinositide 3-kinase (PI3K), phospholipase C (PLC), and SHC. However, the most critical downstream effectors for many physiological functions, particularly in growth hormone signaling, are members of the signal transducer and activator of transcription (STAT) family, which translocate to the nucleus to modulate gene expression.
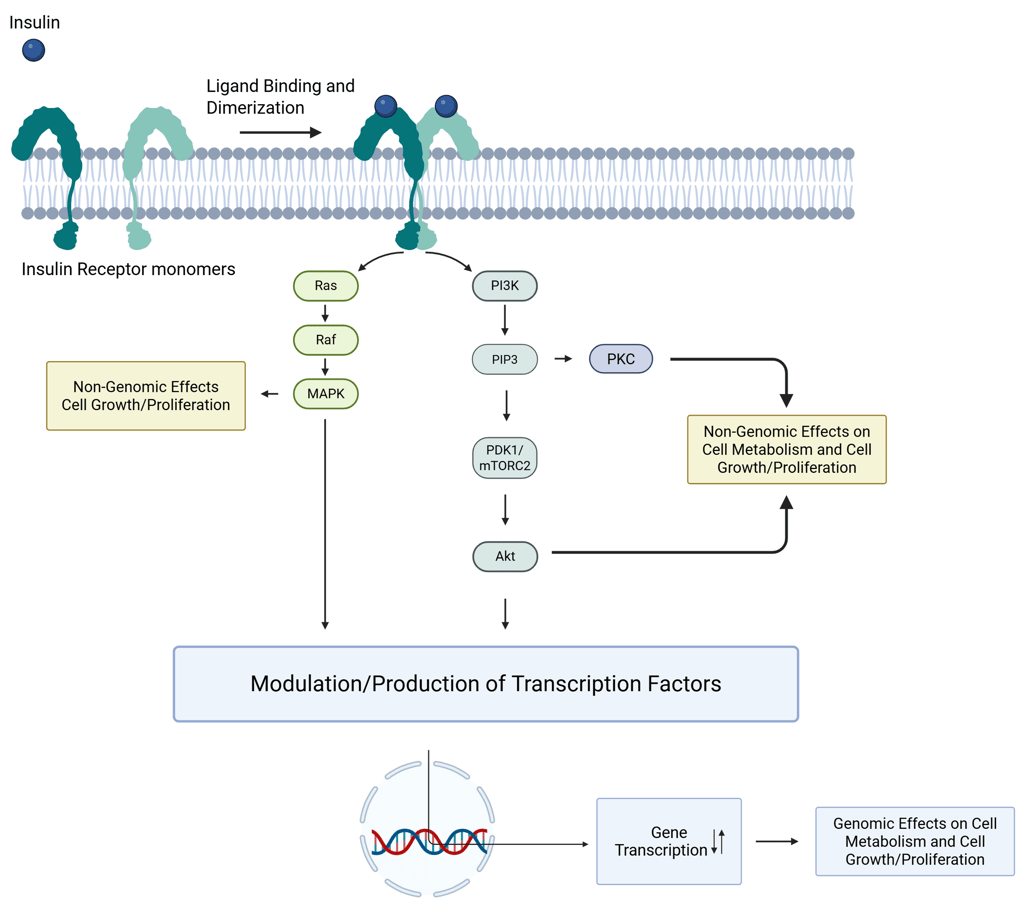
Illustration: Insulin Signaling. One insulin molecule is sufficient to induce dimerization and activation of the receptor. Under saturating conditions, up to four insulin molecules can bind to a single receptor dimer.
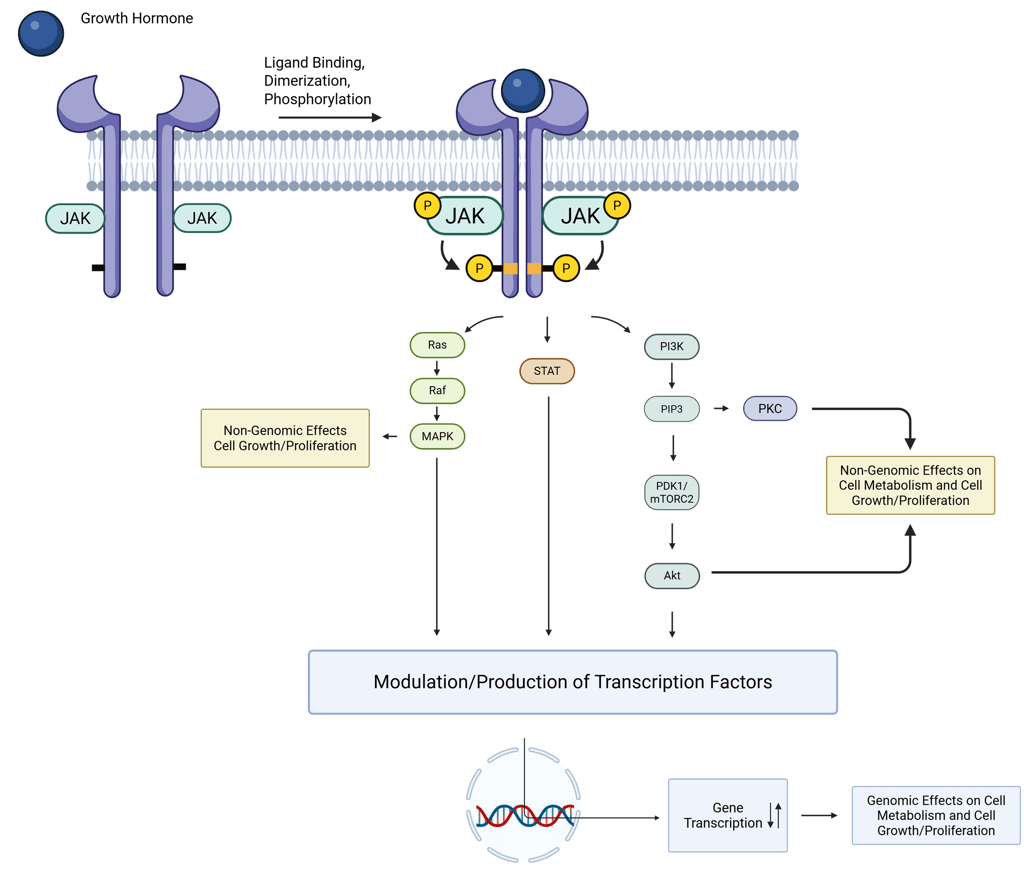
Illustration of GH Signaling
Clinical Relevance. Similar to the GPCR-mediated signaling, the intricate molecular details of tyrosine kinase–mediated signal transduction are not essential for daily clinical decision-making in endocrinology, a fundamental understanding of these pathways is nonetheless highly relevant. It provides critical insight into how a single hormone, such as insulin, can exert multiple, diverse effects across different tissues. Tyrosine kinase–linked receptors, particularly the insulin receptor, are key to mediating both rapid, non-genomic metabolic responses (such as glucose uptake and inhibition of lipolysis) and slower, transcriptional effects that influence growth, differentiation, and protein synthesis. This dual capacity explains the pleiotropic nature of insulin’s actions, ranging from immediate changes in cellular metabolism to long-term regulation of gene expression.
Similarly, hormones like growth hormone and IGF-1, acting through receptors associated with intracellular tyrosine kinases and transcriptional effectors like STAT proteins, demonstrate how signal transduction pathways define both the intensity and duration of hormonal effects. Clinically, this helps contextualize why certain hormone deficiencies or receptor abnormalities may lead to a spectrum of effects rather than a single, isolated dysfunction.
Signaling Through Nuclear Receptors
Numerous signaling molecules, akin to thyroid and steroid hormones, possess the capacity to operate within the nucleus, transmitting intercellular and environmental cues. These molecules are not exclusively synthesized in glandular tissues. While certain signaling agents—such as classical endocrine hormones—reach their target sites via systemic circulation, others act in a paracrine manner, influencing nearby cells, or function autocrinely, affecting the very cell that produced them. In addition to traditional steroid and thyroid hormones, a range of lipophilic molecules—including metabolites derived from vitamins A and D, oxysterols, bile acids, and environmental xenobiotics—engage nuclear receptors as ligands. Collectively referred to as nuclear receptor ligands, these compounds bind to structurally related receptor proteins.
Unlike peptide hormones that initiate signaling through cell surface receptors, nuclear receptor ligands are not genetically encoded. They are small (<1000 Da) and hydrophobic, a property that typically allows them to diffuse freely across the cell membrane, although certain cases require facilitation by membrane transport proteins. For instance, specific and active transporters for thyroid hormones have been identified, including monocarboxylate transporter 8 (MCT8), MCT10, and organic anion-transporting polypeptide 1C1 (OATP1C1).
For a hormone to exert its effect, both the ligand and its corresponding nuclear receptor must be able to access the nucleus. The receptor must also bind its ligand with high affinity to initiate signaling effectively. A key role of nuclear receptors is the selective regulation of gene transcription, which requires precise recognition and binding to specific promoter elements within target genes. One mechanism that contributes to this specificity is receptor dimerization—either as homodimers (two identical receptors; e.g. steroid hormone receptors) or heterodimers (two different receptors; e.g. thyroid hormone receptors with retinoid receptor). Once bound to DNA, the receptor functions within the chromatin environment to influence the basal transcription machinery, thereby modulating gene expression either positively or negatively. While certain core mechanisms of nuclear receptor signaling are conserved across the receptor superfamily, others provide the molecular specificity necessary to account for the diverse physiological actions of the various hormones and ligands that act through these receptors.
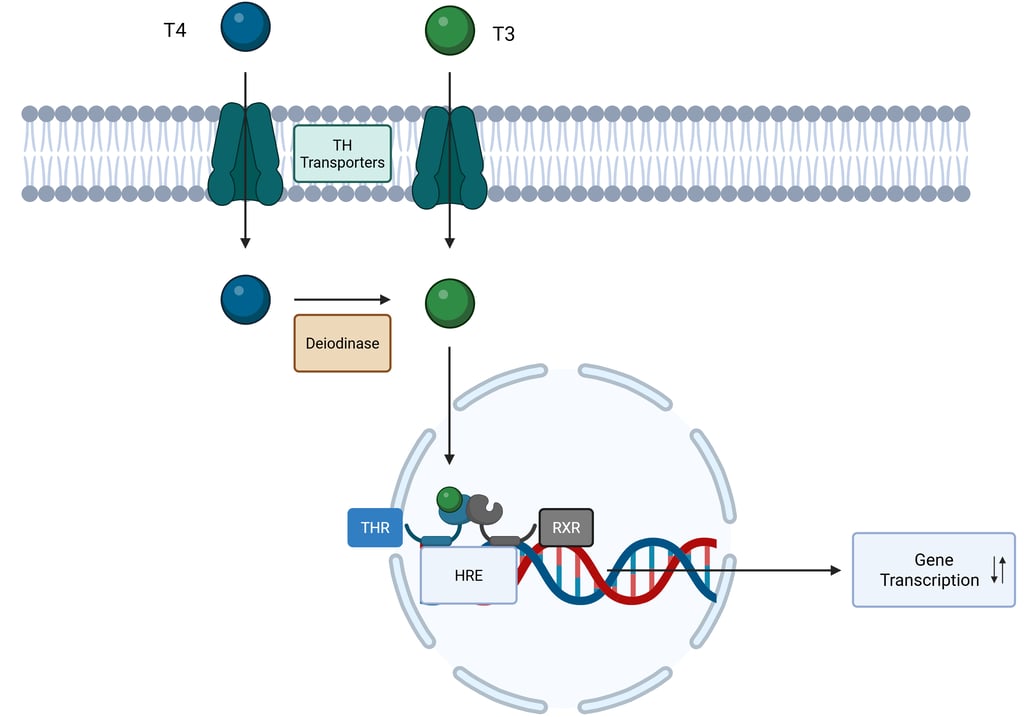
Illustration: Nuclear Receptor Signaling: Thyroid Hormones. THR: Thyroid Hormone Receptor. RXR: Retinoid X Receptor
Through signal transduction, hormones modulate a range of intracellular processes that regulate essential cellular functions. The intracellular effects of hormones on target cells can be broadly categorized into two types:
Genomic Effects: These involve the regulation of gene transcription, typically leading to slower, long-term cellular changes.
Non-Genomic Effects: These involve rapid signaling cascades that induce immediate and faster cellular responses, such as changes in ion transport, enzyme activity, cytoskeletal reorganization, receptor turnover, and other short-term cellular processes.
The combined actions of genomic and non-genomic signaling pathways orchestrated by hormones are fundamental to regulating key cellular processes, including growth, proliferation, differentiation, and metabolism in hormone-responsive tissues. These mechanisms contribute to the maintenance of several vital physiological functions:
Reproduction: Gonadotropins such as follicle-stimulating hormone (FSH) and luteinizing hormone (LH), secreted by the anterior pituitary, are pivotal in coordinating reproductive functions.
Growth and Development: Growth hormone (GH), also from the anterior pituitary, is indispensable for proper physical development, particularly during childhood and adolescence.
Metabolism: Thyroid hormones (T3 and T4) modulate basal metabolic rate, while insulin, produced by the pancreas, plays a central role in glucose uptake and utilization.
Stress Response: The hypothalamic-pituitary-adrenal (HPA) axis regulates the body's adaptation to stress, primarily through the action of cortisol released from the adrenal cortex.
Fluid/ Electrolyte Balance and the Cardiovascular System: Hormonal regulation is essential for maintaining fluid volume, electrolyte concentrations, and vascular tone, thereby supporting cardiovascular stability and blood pressure control.
Circadian Rhythms: Hormones such as cortisol follow circadian patterns, helping to synchronize internal physiological processes with external environmental changes.
The strength and quality of the effects elicited by a hormone-receptor interaction are influenced by several key factors, which include:
Ligand Levels: Target cells must be highly sensitive to very low concentrations of hormones, while maintaining specificity for detecting and responding to the correct signals. Hormone levels at target sites are influenced by:
The availability of precursor molecules to hormone synthesis.
The rate of hormone synthesis and secretion.
The amount of biologically active hormone/Hormone binding proteins
The rate of hormone metabolism, including activation, deactivation, and elimination.
Receptor Activity and Downstream Signaling: The effectiveness of receptor activation and its downstream signaling cascade is influenced by:
Cell Specificity: For hormone signaling to occur, the target cell must express specific receptors capable of binding the hormone. The presence or absence of these receptors is a fundamental determinant of cellular responsiveness.
Receptor Density: Higher receptor availability generally leads to a stronger response. This depends on the synthesis and clearance rates of the receptors, processes, that are highly regulated.
Receptor Binding and Affinity: Different receptors may bind the same hormone with varying affinities. For example, norepinephrine binds to both alpha and beta-adrenergic receptors, but with higher affinity to the alpha receptor.
Presence of Competing Ligands: Other molecules can compete for receptor binding, potentially altering the response.
Efficiency of Downstream Signaling: The effectiveness of intracellular signaling cascades initiated by receptor activation can modulate the cellular response.
Tissue Specificity: The effects of hormones can vary significantly depending on the tissue type. Different tissues may express distinct receptor subtypes or co-regulatory molecules, which can influence the sensitivity and magnitude of the response to a hormone.
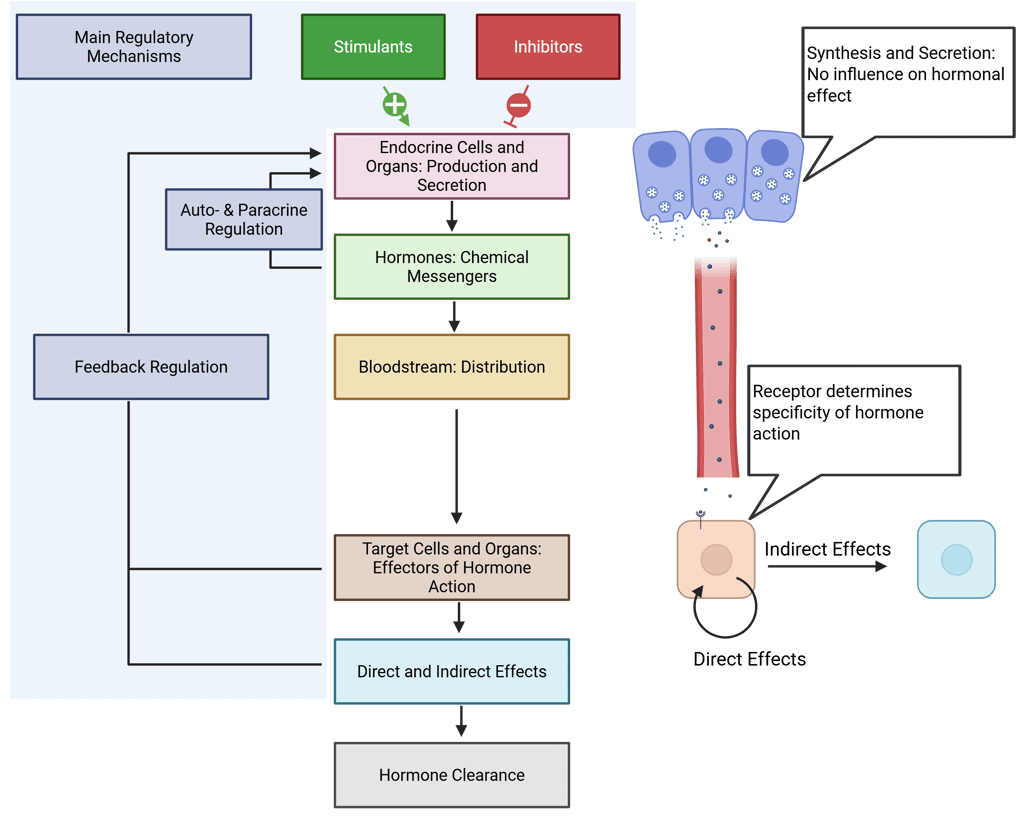
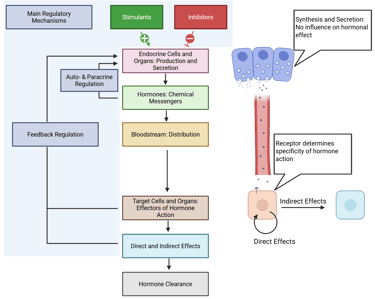
Illustration: Basic Principles of Endocrine Systems and Determination of Hormone Effect
References
All Illustrations were created in https://BioRender.com
For References, visit the Section "References" in General Principles of Clinical Endocrinology
© 2025 EndoCases. All rights reserved.
This platform is intended for medical professionals, particularly endocrinology residents, and is provided for educational purposes only. It supports learning and clinical reasoning but is not a substitute for professional medical advice or patient care. The information is general in nature and should be applied with appropriate clinical judgment and in accordance with local guidelines.
Portions of the text on this website were edited with the assistance of Artificial Intelligence to improve grammar and phrasing, as English is not my first language. All medical content, ideas for illustrations, and overall structure are original and based on the author’s own expertise and the cited medical literature. No AI tools were used to generate or influence the educational content itself.
All of the content is independent of my employer.
Use of this site implies acceptance of our Terms of Use
Contact us via E-Mail: contact@endo-cases.com